Chromatographic purity—
Buffer solution—
Dissolve 3.25 g of 1-octanesulfonic acid sodium salt monohydrate in 1 L of water. Adjust slowly with 3 M phosphoric acid to a pH of 2.8.
Solution A—
Prepare a filtered and degassed mixture of Buffer solution and acetonitrile (9:1).
Solution B—
Prepare a filtered and degassed mixture of acetonitrile and Buffer solution (9:1).
Mobile Phase—
Use variable mixtures of
Solution A and
Solution B as directed for
Chromatographic system. Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Diluent—
Prepare a mixture of Solution A and Solution B (8:2).
Blank solution—
Prepare a solution containing 0.8 mg per mL L(+)-tartaric acid in Diluent.
Test solution—
Transfer 78 mg of Phenylephrine Bitartrate, accurately weighed, to a 50-mL volumetric flask. Dissolve in and dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 215-nm detector and a 4-mm × 5.5-cm column that contains packing L1. The column and injection port temperatures are maintained at 45 ± 2
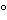
. The flow rate is about 1.5 mL per minute. The chromatograph is programmed as follows.
Time
(minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 |
93 |
7 |
equilibration |
| 0–10 |
93®70 |
7®30 |
linear gradient |
| 10–10.1 |
70®93 |
30®7 |
linear gradient |
| 10.1–18 |
93 |
7 |
equilibration |
Chromatograph the
System suitability solution, and record the peak responses as directed for
Procedure: the resolution,
R, between norphenylephrine and (–)-phenylephrine is not less than 1.5; the tailing factor of (–)-phenylephrine is less than 1.8; and the relative standard deviation for replicate injections is not more than 5%.
Procedure—
Separately inject equal volumes (about 4 µL) of the
Blank solution and the
Test solution into the chromatograph, record the chromatograms, and measure all of the peak responses. Calculate the percentage of each impurity in the portion of Phenylephrine Bitartrate taken by the formula:
100(ri / rs),
in which
ri is the peak response for each impurity, and
rs is the sum of the responses of all the peaks.
[NOTE—Examine the chromatogram of the
Blank solution for peaks and disregard any corresponding peaks observed in the chromatogram of the
Test solution.] The limits of impurities are specified in the accompanying table.
| Compound |
Approximate Relative Retention Time |
Limit (%) |
| Phenylephrine |
1.0 |
— |
| Norphenylephrine |
0.9 |
0.2 |
| Phenylephrone |
1.2 |
0.1 |
| Benzylphenylephrine |
2.9 |
0.2 |
| Benzylphenylephrone |
3.1 |
0.1 |
Individual unknown
 impurity impurity |
— |
0.2 |
| Total impurity |
— |
0.5 |
Assay—
Transfer about 280 mg of Phenylephrine Bitartrate, accurately weighed, to a 100-mL beaker, and dissolve by stirring in 60 mL of glacial acetic acid. Titrate with 0.1 N perchloric acid, determining the endpoint potentiometrically. Perform a blank determination (see
Titrimetry  541
541
), and make the necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 31.73 mg of C
9H
13NO
2 · C
4H
6O
6.

