Labeling—
Label it to indicate the number of mL of solution required per 100 mL of whole blood or the number of mL of solution required per volume of whole blood to be collected.
Chloride  221
221 —
—
A 10-mL portion shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid (0.0035%).
Other requirements—
It meets the requirements under
Injections  1
1
.
Assay for total citrate—
Standard preparations—
Dissolve a suitable quantity of citric acid, previously dried at 90
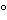
for 3 hours and accurately weighed, in water to obtain a solution having a known concentration of about 1.0 mg of anhydrous citric acid per mL. Further pipet quantities of 8, 9, 10, 11, and 12 mL of this stock standard into separate 100-mL volumetric flasks, dilute with water to volume, and mix.
Assay preparation—
Pipet 5 mL of Solution into a 1000-mL volumetric flask, dilute with water to volume, and mix.
Procedure—
Pipet 1 mL of the
Assay preparation, each
Standard preparation, and water into separate test tubes. To each tube add 1.3 mL of pyridine, and mix by swirling. To one tube at a time add 5.7 mL of acetic anhydride, and mix, using a rotary vortex stirrer. Immediately place in a water bath maintained at 31 ± 1.0
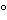
, and allow the color to develop for 33 ± 1 minutes. Determine the absorbance against the reference blank in 1-cm cells at 425 nm, taking care to measure the absorbance of each solution at the same elapsed time from mixing. Calculate the total citrate content, in mg per mL, of the Solution taken by the formula:
0.2C,
in which
C is the concentration, in µg per mL, of anhydrous citric acid read from the standard curve.
Assay for total phosphate
[expressed as monobasic sodium phosphate monohydrate (NaH
2PO
4·H
2O)]—
Acid solution—
Dilute 75 mL of sulfuric acid to 200 mL with water.
Ammonium molybdate solution—
Dissolve 25 g of ammonium molybdate in 300 mL of water, and add 200 mL of Acid solution.
Standard preparation—
Dissolve about 0.44 g of monobasic potassium phosphate, previously dried at 105
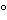
for 4 hours and accurately weighed, in water to make 1000 mL, and mix. Pipet 10 mL of this solution into a 100-mL volumetric flask, dilute with water to volume, and mix to obtain a solution having a known concentration of about 0.01 mg of phosphorus (P) per mL.
Assay preparation—
Pipet 10 mL of Solution into a 500-mL volumetric flask, dilute with water to volume, and mix.
Procedure—
Pipet 5-, 10-, and 15-mL portions of the
Standard preparation and 10 mL of the
Assay preparation into separate 50-mL volumetric flasks. Treat the contents of each flask as follows. Add 2.5 mL of
Ammonium molybdate solution, and mix. Add rapidly, and in order, 2.5 mL of hydroquinone solution (1 in 200) and 2.5 mL of sodium sulfite solution (1 in 10), both prepared fresh daily. Dilute with water to volume, mix, and allow to stand at room temperature for 30 ± 5 minutes. Determine the absorbances against water in 1-cm cells at 660 nm with a suitable spectrophotometer. Plot the absorbances against the mg of phosphorus in the portions of
Standard preparation taken. Calculate the quantity, in mg, of NaH
2PO
4·H
2O in each mL of the Solution taken by the formula:
(137.99/30.9738)(5W),
in which 137.99 is the molecular weight of monobasic sodium phosphate monohydrate; 30.9738 is the atomic weight of phosphorus; and
W is the weight, in mg, of P in the 10 mL of
Assay preparation taken, read from the Standard curve.
Assay for sodium—
Lithium diluent solution—
Transfer 1.04 g of lithium nitrate to a 1000-mL volumetric flask, add a suitable nonionic surfactant, then add water to volume, and mix. This solution contains 15 mEq of lithium per L.
Standard preparation—
Transfer 8.18 g of sodium chloride, previously dried at 105
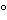
for 2 hours and accurately weighed, to a 1000-mL volumetric flask, dilute with water to volume, and mix. This solution contains 140 mEq of sodium per L. Transfer 50 µL of this solution to a 10-mL volumetric flask, dilute with
Lithium diluent solution to volume, and mix.
Assay preparation—
Pipet 25 mL of Solution into a 50-mL volumetric flask, dilute with water to volume, and mix. Transfer 50 µL of this solution to a 10-mL volumetric flask, dilute with Lithium diluent solution to volume, and mix.
Procedure—
Using a suitable flame photometer, adjusted to read zero with
Lithium diluent solution, concomitantly determine the sodium flame emission readings for the
Standard preparation and the
Assay preparation at the wavelength of maximum emission at about 589 nm. Calculate the quantity, in g, of Na in 1000 mL of Anticoagulant Citrate Phosphate Dextrose Adenine Solution taken by the formula:
2(8.18)(22.99/58.44)(RU/RS),
in which 8.18 is the weight, in g, of sodium chloride taken to make the
Standard preparation; 22.99 is the atomic weight of sodium; 58.44 is the molecular weight of sodium chloride; and
RU and
RS are the sodium emission readings obtained from the
Assay preparation and the
Standard preparation, respectively.
Assay for dextrose—
Tare a clean, medium-porosity filtering crucible containing several carborundum boiling chips or glass beads. Pipet 50 mL of freshly mixed alkaline cupric tartrate TS into a 400-mL beaker. Add the boiling chips or glass beads from the tared crucible, 45 mL of water, and 5.0 mL of Solution to the beaker. Heat the beaker and contents over a burner that has been adjusted to cause boiling of the solution to start in 3.5 to 4 minutes. Boil the solution for 2 minutes, accurately timed, and filter immediately through the tared crucible, taking care to transfer all of the boiling chips or glass beads to the crucible. Wash the precipitate with hot water and 10 mL of alcohol. Dry the crucible and contents at 110
to constant weight. Perform a blank determination, and make any necessary correction. Each mg of cuprous oxide precipitate obtained is equivalent to 0.496 mg of C
6H
12O
6·H
2O.
Assay for adenine—
Mobile phase—
Dissolve 3.45 g of ammonium dihydrogen phosphate in 950 mL of water in a 1000-mL volumetric flask, add 10 mL of glacial acetic acid, dilute with water to volume, mix, pass through a membrane filter having a 1-µm or finer porosity, and degas.
Standard preparations—
Dissolve accurately weighed quantities of
USP Adenine RS in dilute hydrochloric acid (1 in 120) in three separate volumetric flasks, dilute with the dilute hydrochloric acid solution to volume, and mix to obtain Standard preparations having known concentrations of about 0.25, 0.275, and 0.30 mg of adenine per mL, respectively. Protect from light.
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm × 30-cm stainless steel column that contains packing L9. The flow rate is about 2.0 mL per minute. Prepare a solution containing
USP Adenine RS and purine, each at about 0.275 mg per mL, in dilute hydrochloric acid (1 in 120), and chromatograph not less than four injections (about 20 µL) of this solution: the relative standard deviation of the peak response of adenine is not more than 2.5%, the relative standard deviation of the retention time of adenine is not more than 2.0%, and the resolution of adenine and purine is not less than 3.0.
Procedure—
Separately inject equal volumes (about 20 µL) of the Solution and the
Standard preparations, record the chromatograms, and measure the responses for the major peaks. Plot the responses against the concentrations, in mg of
USP Adenine RS per mL of the
Standard preparations. Calculate the quantity, in mg, of C
5H
5N
5 in each mL of the Solution taken as the value read directly from the Standard curve corresponding to the response obtained from the portion of the Anticoagulant Citrate Phosphate Dextrose Adenine Solution chromatographed.
Auxiliary Information—
Staff Liaison :
Andrzej Wilk, Ph.D., Senior Scientific Associate
Expert Committee : (BBBBP05) Biologics and Biotechnology - Blood and Blood Products
USP29–NF24 Page 182
Pharmacopeial Forum : Volume No. 31(3) Page 728
Phone Number : 1-301-816-8305

