C
17H
25N
3O
5S·3H
2O





437.52
1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 3-[[5-[(dimethylamino)carbonyl]-3-pyrrolidinyl]thio]-6-(1-hydroxyethyl)-4-methyl-7-oxo, trihydrate, [4
R-[3(3
S*,5
S*),4
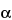
,5
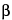
,6
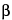
(
R*)]]-.
(4
R,5
S,6
S)-3-[[(3
S,5
S)-5-(Dimethylcarbamoyl)-3-pyrrolidinyl]thio]-6-[(1
R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-carboxylic acid, trihydrate



[
119478-56-7].
Anhydrous
383.47



[
96036-03-2].
Packaging and storage—
Preserve in tight containers. Store the dry powder at controlled room temperature.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
pH  791
791 :
:
between 4.0 and 6.0, in a solution (1 in 100).
Residue on ignition  281
281 :
:
not more than 0.1%, igniting at 500 ± 50

, instead of at 800 ± 25

. Use a desiccator containing silica gel.
Heavy metals—
Sodium sulfide reagent—
Dissolve 5 g of sodium sulfide in a mixture of 10 mL of water and 30 mL of glycerin. Preserve in well-filled, light-resistant bottles, and use within 3 months.
Test solution—
Transfer 1.0 g of Meropenem to a quartz or porcelain crucible, cover loosely with a lid, and carbonize by gentle ignition. After cooling, add 2 mL of nitric acid and 5 drops of sulfuric acid, heat cautiously until white fumes evolve, and incinerate by ignition at 500

to 600

. Cool, add 2 mL of hydrochloric acid, and evaporate on a water bath to dryness. Moisten the residue with 3 drops of hydrochloric acid, add 10 mL of hot water, and warm for 2 minutes. Add 1 drop of phenolphthalein TS, add
ammonia TS, dropwise, until the solution develops a pale red color, and add 2 mL of 1 N acetic acid. Filter, if necessary, to obtain a clear solution, washing the filter with 10 mL of water. Transfer the filtrate and the washing to a 50-mL color-comparison tube, and add water to obtain a volume of 50 mL.
Standard solution—
Evaporate a mixture of 2 mL of nitric acid, 5 drops of sulfuric acid, and 2 mL of hydrochloric acid on a water bath, further evaporate to dryness on a hot sand bath, and moisten the residue with 3 drops of hydrochloric acid. Proceed as directed for
Test solution, beginning with “add 10 mL of hot water,” except add water to obtain a volume of 49 mL. Add 1.0 mL of
Standard Lead Solution (see
Heavy Metals  231
231
).
Procedure—
To the tubes containing the Test solution and the Standard solution, add 1 drop of Sodium sulfide reagent, mix, and allow to stand for 5 minutes. The color in the tube containing the Test solution is not darker than the color in the tube containing the Standard solution (0.001%).
Limit of acetone—
Internal standard solution—
Prepare a solution in dimethylformamide containing 0.05 µL of ethyl acetate per mL.
Standard solution—
Transfer about 50 mg of acetone, accurately weighed, to a 100-mL volumetric flask, dilute with dimethylformamide to volume, and mix. To 1.0 mL of this solution, add 10.0 mL of the Internal standard solution, and mix.
Test solution—
Dissolve 100 mg of Meropenem, accurately weighed, in 0.2 mL of dimethylformamide and 2.0 mL of Internal standard solution.
Chromatographic system (see Chromatography  621
621 )—
)—
The gas chromatograph is equipped with a flame-ionization detector and a 3-mm × 2-m column that contains support S2 and is maintained at a constant temperature of about 150

. The injection port temperature is maintained at about 170

. Nitrogen is the carrier gas, with the flow rate adjusted so that the retention time for acetone is about 3 minutes.
Procedure—
Separately inject equal volumes (about 2 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatograms, and measure the peak responses for the acetone peak and the internal standard peak. Calculate the percentage of acetone in the portion of Meropenem taken by the formula:
(WA/5WU)(RU / RS)
in which
WA is the weight, in mg, of acetone in the
Standard solution; WU is the quantity, in mg, of Meropenem in the
Test solution; and
RU and
RS are the peak area ratios of acetone to the internal standard obtained from the
Test Solution and the
Standard solution, respectively. Not more than 0.05% is found.
Chromatographic purity—
Diluted phosphoric acid—
Dilute 10 mL of phosphoric acid with water to make 100 mL of solution.
Solvent—
Transfer 1.0 mL of triethylamine to a 1000-mL volumetric flask containing 900 mL of water. Adjust with Diluted phosphoric acid to a pH of 5.0 ± 0.1, dilute with water to volume, and mix.
Mobile phase—
Transfer 1.0 mL of triethylamine to a 1000-mL volumetric flask containing 900 mL of water. Adjust with
Diluted phosphoric acid to a pH of 5.0 ± 0.1, dilute with water to volume, and mix. Mix this solution with 70 mL of acetonitrile. Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard solution—
Prepare a solution of
USP Meropenem RS in
Solvent having a known concentration of about 0.025 mg of
USP Meropenem RS per mL.
[note—Immediately after preparation, store this solution in a refrigerator and use within 24 hours.
]
Test solution—
Dissolve an accurately weighed quantity of Meropenem quantitatively in Solvent to obtain a solution having a known concentration of about 5 mg per mL. Use this Test solution immediately.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1 and is maintained at a constant temperature of about 40

. The flow rate is about 1.6 mL per minute, and is adjusted so that the retention time of meropenem is between 5 and 7 minutes. Chromatograph the
Standard solution, and record the peak responses as directed for
Procedure: the column efficiency is not less than 2500 theoretical plates; the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatograms, using a period of chromatography for the
Test solution that is about 3 times the retention time of meropenem, and measure the peak responses. Major impurity peaks may be observed at retention times of about 0.45 and 1.9 in relation to the retention time of meropenem. Calculate the percentage of each impurity in the chromatogram obtained from the
Test solution by the formula:
(CS / CU)(P)(ri / rS)
in which
CS is the concentration, in mg per mL, of
USP Meropenem RS in the
Standard solution; CU is the concentration, in mg per mL, of Meropenem in the
Test solution; P is the stated percentage, calculated on the anhydrous basis, of meropenem in
USP Meropenem RS;
ri is the peak response of any individual impurity obtained from the
Test solution; and
rS is the peak response of meropenem obtained from the
Standard solution. Not more than 0.3% of any of two major impurities is found, calculated on the anhydrous basis; not more than 0.1% of any other impurity is found, calculated on the anhydrous basis; and the sum of all such other impurities is not more 0.3%.
Other requirements—
Where the label states that Meropenem is sterile, it meets the requirements for
Sterility  71
71
and for
Bacterial endotoxins under
Meropenem for Injection. Where the label states that Meropenem must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for
Bacterial endotoxins under
Meropenem for Injection.
Assay—
Diluted phosphoric acid—
Dilute 10 mL of phosphoric acid with water to make 100 mL of solution.
Solvent—
Transfer 1.0 mL of triethylamine to a 1000-mL volumetric flask containing 900 mL of water. Adjust with Diluted phosphoric acid to a pH of 5.0 ± 0.1, dilute with water to volume, and mix.
Mobile phase—
Prepare a mixture of
Solvent and methanol (5:1). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard preparation—
Transfer about 25 mg of
USP Meropenem RS, accurately weighed, to a 50-mL volumetric flask, add
Solvent, swirl to dissolve, dilute with
Solvent to volume, and mix.
[note—Immediately after preparation, store this solution in a refrigerator. It may be used for 24 hours.
]
Assay preparation—
Transfer about 25 mg of Meropenem, accurately weighed, to a 50-mL volumetric flask, add Solvent, swirl to dissolve, dilute with Solvent to volume, and mix. Use this solution immediately after preparation.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 300-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. Adjust the flow rate so that the retention time for meropenem is about 6 to 8 minutes. The flow rate is about 1.5 mL per minute. Chromatograph the
Standard preparation, and record the peak responses as directed for
Procedure: the column efficiency is not less than 2500 theoretical plates; the tailing factor is not more than 1.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 5 µL) of
Standard preparation and
Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in mg, of C
17H
25N
3O
5S in the portion of Meropenem taken by the formula:
(WS/WU)(P)(rU / rS)
in which
WS is the weight, in mg, of
USP Meropenem RS taken to prepare the
Standard preparation, calculated on the anhydrous basis;
WU is the weight, in mg, of Meropenem taken to prepare the
Assay preparation; P is the stated percentage, calculated on the anhydrous basis, of meropenem in
USP Meropenem RS; and
rU and
rS are the meropenem peak responses obtained from the
Assay preparation and the
Standard preparation, respectively.
Auxiliary Information—
Staff Liaison :
Ahalya Wise, M.S. , Scientist
Expert Committee : (MDANT05) Monograph Development-Antibiotics
USP31–NF26 Page 2627
Pharmacopeial Forum : Volume No. 27(1) Page 1801
Phone Number : 1-301-816-8161

