Limit of 1,2,3-trimercaptopropane and related impurities—
Adsorbent—
Use a suitable chromatographic grade of 100-mesh silicic acid.
Standard buffer solution—
Prepare 100 mL of pH 6.0 Phosphate Buffer (see
pH  791
791
), and dissolve in it 100 mg of sodium bisulfite.
Acid-washed solvent hexane—
To 100 mL of solvent hexane contained in a separator add 10 mL of sulfuric acid, shake for not less than 12 hours, and allow the layers to separate. Transfer the acid-washed solvent to a distilling flask, and distill slowly, retaining only that portion distilling between 35
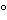
and 50
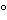
. Use only freshly distilled material.
Diisopropyl ether—
Place 100 mL of diisopropyl ether in a distilling flask, and distil, retaining only that portion distilling between 68
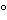
and 69
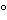
. Use only freshly distilled material.
[Caution—Do not evaporate to the point of near-dryness, since diisopropyl ether tends to form explosive peroxides
]
Solvent hexane-diisopropyl ether mixture
(mobile solvent)—Mix 50 mL of Diisopropyl ether with 50 mL of Acid-washed solvent hexane.
Chromatographic tube—
Insert a small plug of glass wool at the juncture of the tube and the stem of a 600- × 13-mm chromatographic tube.
Chromatographic column—
Mix 20 g of Adsorbent with 20 mL of Standard buffer solution. Make into a slurry by mixing with 100 mL of chloroform. Transfer successive portions of the slurry into the Chromatographic tube, packing firmly and evenly with a close-fitting, ground-glass tamper after each addition. Keep a layer of liquid above the packed column to prevent the formation of air spaces. Wash the column free from chloroform with Solvent hexane-diisopropyl ether mixture, and allow the solvent to fall to the level of the Adsorbent.
Procedure—
Place about 250 mg of Dimercaprol, accurately weighed and demonstrated to be free from hydrogen sulfide as directed in the
Assay, in a 5-mL volumetric flask, add
Solvent hexane-diisopropyl ether mixture to volume, and mix. Transfer 2.0 mL of the resulting solution to the prepared
Chromatographic column. When the liquid has passed into the column, wash the wall of the tube with a 2-mL portion of
Solvent hexane-diisopropyl ether mixture, and allow the washing to fall to the level of the
Adsorbent. Fill the
Chromatographic tube with solvent, and collect two successive fractions: (
A) a 20-mL fraction containing all of the 1,2,3-trimercaptopropane, and (
B) a 3-mL fraction that serves as a check on the separation. To each fraction add an equal volume of alcohol, and titrate with 0.1 N iodine VS until a permanent yellow color is produced. Perform a blank titration on 20 mL of the solvent mixture that has been passed through the column prior to introduction of the test specimen, and make any necessary correction. Fraction (
B) does not decolorize 1 drop of 0.1 N iodine VS. Each mL of 0.1 N iodine added is equivalent to 4.676 mg of C
3H
8S
3. Not more than 1.5% of 1,2,3-trimercaptopropane (C
3H
8S
3) is found.
Assay—
Test the Dimercaprol for the presence of hydrogen sulfide by examining the vapor above the assay specimen with moistened lead acetate test paper. If the paper darkens, bubble dry, oxygen-free nitrogen or carbon dioxide through the assay specimen until a fresh strip of test paper gives a negative test. Transfer about 2 mL of hydrogen sulfide-free Dimercaprol to a tared, glass-stoppered, 100-mL volumetric flask, weigh accurately, add methanol to volume, and mix. Pipet 10 mL of the solution into a 50-mL conical flask, and titrate with 0.1 N iodine VS until a permanent yellow color is produced. Perform a blank titration, and make any necessary correction. Calculate the percentage of C
3H
8OS
2 taken by the formula:
0.6211
V/
W 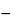
1.328
T,
in which
V is the volume, in mL, of 0.1 N iodine used,
W is the weight, in g, of specimen in the aliquot taken, and
T is the percentage of C
3H
8S
3 found in the determination of the
Limit of 1,
2,
3-trimercaptopropane and related impurities.

