Loss on drying 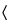 731
731 —
—
Dry about 100 mg, accurately weighed, in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60
for 3 hours: it loses not more than 1.0% of its weight.
Permeability diameter—
Determine the apparent particle size in µm by the air-permeation method, using a suitable subsieve sizer. Weigh 1.819 ± 0.001 g of Griseofulvin, and transfer to the compression tube of the apparatus. Compact with moderate pressure so that a uniform porosity is achieved. Pass dry compressed air through the tube, and measure the air pressure with a water manometer. Read the porosity, and calculate the apparent particle size from the instrument equation. Repeat the porosity readings at successively higher degrees of compaction until the apparent particle size reaches a minimum value. Calculate the observed permeability diameter, in square meters per g, taken by the formula:
6 / (1.455MF),
in which
M is the minimum apparent particle size; and
F is a factor, obtained from the accompanying table, interpolation being used if necessary, to correct the apparent particle size to the true particle size at a given porosity reading.
Porosity
Reading |
F |
Porosity
Reading |
F |
| 0.80 |
1.3771 |
0.56 |
1.7353 |
| 0.76 |
1.4142 |
0.52 |
1.8528 |
| 0.72 |
1.4573 |
0.48 |
2.0076 |
| 0.68 |
1.5082 |
0.44 |
2.2203 |
| 0.64 |
1.5690 |
0.40 |
2.5298 |
| 0.60 |
1.6432 |
|
|
Concomitantly determine the observed permeability diameter of a similar preparation of
USP Griseofulvin Permeability Diameter RS. Calculate the permeability diameter of the Griseofulvin taken by the formula:
OU (AS / OS),
in which
OU is the observed permeability diameter of the specimen,
AS is the assigned permeability diameter of
USP Griseofulvin Permeability Diameter RS, and
OS is the observed permeability diameter of
USP Griseofulvin Permeability Diameter RS: it is between 1.3 and 1.7 square meters per g.
Assay—
Mobile phase—
Prepare a suitable filtered mixture of water, acetonitrile, and tetrahydrofuran (60:35:5). Degas for 5 minutes before use, and stir continuously during use. Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard preparation—
Dissolve an accurately weighed quantity of
USP Griseofulvin RS in methanol to obtain a solution having a known concentration of about 1.25 mg per mL. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, dilute with
Mobile phase to volume, and mix. This solution contains about 0.125 mg of
USP Griseofulvin RS in each mL.
Assay preparation—
Transfer about 62 mg of Griseofulvin, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with methanol to volume, and mix. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L10. The flow rate is about 1 mL per minute. The relative standard deviation for replicate injections of
Standard preparation is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the
Standard preparation and the
Assay preparation into the chromatograph, and measure the peak responses for the major peaks. Calculate the quantity, in µg of C
17H
17ClO
6, in each mg of the Griseofulvin taken by the formula:
500(CP / WU)(rU / rS),
in which
C is the concentration, in mg per mL, of
USP Griseofulvin RS in the
Standard preparation; P is the content, in µg of C
17H
17ClO
6 per mg, of
USP Griseofulvin RS;
WU is the quantity, in mg, of Griseofulvin taken; and
rU and
rS are the griseofulvin peak responses obtained from the
Assay preparation and the
Standard preparation, respectively.

