Selective Cleavage of Peptide Bonds
The selection of the approach used for the cleavage of peptide bonds will depend on the protein under test. This selection process involves determination of the type of cleavage to be employed—enzymatic or chemical—and the type of cleavage agent within the chosen category. Several cleavage agents and their specificity are shown in
Table 1.
Table 1. Examples of Cleavage Agents
| Type |
Agent |
Specificity |
| Enzymatic |
Trypsin, EC 3.4.21.4 |
C-terminal side of Arg and Lys |
|
Chymotrypsin, EC 3.4.21.1 |
C-terminal side of hydrophobic residues (e.g., Leu, Met, Ala, aromatics) |
|
Pepsin A (Pepsin), EC 3.4.23.1 |
Nonspecific digest |
|
Lysyl endopeptidase (Lys-C endopeptidase), EC 3.4.21.50 |
C-terminal side of Lys |
|
Glutamyl endopeptidase (Glu-C endoproteinase; V8 protease); (from S. aureus strain V8), EC 3.4.21.19 |
C-terminal side of Glu and Asp |
|
Peptidyl-Asp metalloendopeptidase (Asp-N endoproteinase), EC 3.4.24.33 |
N-terminal side of Asp |
|
Clostripain (Arg-C endopeptidase), EC 3.4.22.8 |
C-terminal side of Arg |
| Chemical |
Cyanogen bromide |
C-terminal side of Met |
|
2-Nitro-5-thiocyanobenzoic acid |
N-terminal side of Cys |
|
O-Iodosobenzoic acid |
C-terminal side of Trp and Tyr |
|
Dilute acid |
Asp and Pro |
|
BNPS-skatole |
Trp |
This list is not all-inclusive and will be expanded as other cleavage agents are identified.
Pretreatment of Sample—
Depending on the size or the configuration of the protein, different approaches in the pretreatment of samples can be used. For monoclonal antibodies, the heavy and light chains will need to be separated before mapping. If trypsin is used as a cleavage agent for proteins with a molecular mass greater than 100,000 Da, lysine residues must be protected by citraconylation or maleylation; otherwise, too many peptides will be generated.
Pretreatment of the Cleavage Agent—
Pretreatment of cleavage agents, especially enzymatic agents, might be necessary for purification purposes to ensure reproducibility of the map. For example, trypsin used as a cleavage agent will have to be treated with tosyl-l-phenylalanine chloromethyl ketone to inactivate chymotrypsin. Other methods, such as purification of trypsin by HPLC or immobilization of enzyme on a gel support, have been successfully used when only a small amount of protein is available.
Pretreatment of the Protein—
It might be necessary under certain conditions to concentrate the sample, or to separate the protein from added substances and stabilizers used in the formulation of the product if these interfere with the mapping procedure. Physical procedures used for pretreatment can include ultrafiltration, column chromatography, and lyophilization.
Other pretreatments such as the addition of chaotropic agents (e.g., urea) can be used to unfold the protein prior to mapping. It is often necessary to reduce and alkylate the disulfide bonds prior to digestion in order to allow the enzyme to have full access to cleavage sites and permit some unfolding of the protein.
Digestion with trypsin can introduce ambiguities in the tryptic map as a result of side reactions occurring during the digestion reaction, such as nonspecific cleavage, deamidation, disulfide isomerization, oxidation of methionine residues, or formation of pyroglutamic groups created from the deamidation of glutamine at the N-terminal side of a peptide. Furthermore, peaks may be produced by autohydrolysis of trypsin. Their intensities depend on the ratio of trypsin to protein. To avoid autohydrolysis, solutions of proteases may be prepared at a pH that is not optimal (e.g., at pH 5 for trypsin), which would mean that the enzyme would not become active until diluted with the digest buffer.
Establishment of Optimal Digestion Conditions—
Factors that affect the completeness and effectiveness of digestion of proteins are those that could affect any chemical or enzymatic reactions.
pH—
The digestion mixture pH is empirically determined to ensure the optimal performance of the given cleavage agent. For example, a highly acidic environment (e.g., pH 2, formic acid) is necessary when using cyanogen bromide as a cleavage agent; however, a slightly alkaline environment (pH 8) is optimal when using trypsin as a cleavage agent. As a general rule, the pH of the reaction milieu should not alter the chemical integrity of the protein during the digestion and should not change during the course of the fragmentation reaction.
Temperature—
A temperature between 25

and 37

is adequate for most digestions. The temperature used is intended to minimize chemical side reactions. The type of protein under test will dictate the temperature of the reaction milieu because some proteins are more susceptible to denaturation as the temperature of the reaction increases. For example, digestion of recombinant bovine somatropin is conducted at 4

because at higher temperatures it will precipitate during digestion.
Time—
If a sufficient amount of sample is available, a time course study is considered in order to determine the optimum time to obtain a reproducible map and avoid incomplete digestion. Time of digestion varies from 2 to 30 hours. The reaction is stopped by the addition of an acid that does not interfere with the tryptic map, or by freezing.
Amount of Cleavage Agent—
Although excessive amounts of cleavage agent are used to accomplish a reasonably rapid digestion time (i.e., 6 to 20 hours), the amount of cleavage agent is minimized to avoid its contribution to the chromatographic map pattern. A protein-to-protease ratio between 20:1 and 200:1 is generally used. It is recommended that the cleavage agent be added in two or more stages to optimize cleavage. Nonetheless, the final reaction volume remains small enough to facilitate the next step in peptide mapping—the separation step. To sort out digestion artifacts that might be interfering with the subsequent analysis, a blank determination is performed using a digestion control with all the reagents except the test protein.
Chromatographic Separation
Many techniques are used to separate peptides for mapping. The selection of a technique depends on the protein being mapped. Techniques that have been successfully used for the separation of peptides are shown in
Table 2.
Table 2. Techniques Used for the Separation of Peptides
| Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) |
| Ion-Exchange Chromatography (IEC) |
| Hydrophobic Interaction Chromatography (HIC) |
| Polyacrylamide Gel Electrophoresis (PAGE), nondenaturating |
| Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) |
| Capillary Electrophoresis (CE) |
| Paper Chromatography |
| High-Voltage Paper Electrophoresis (HVPE) |
In this section, a most widely used reverse-phase HPLC (RP-HPLC) method is described as one of the procedures of chromatographic separation.
The purity of solvents and mobile phases is a critical factor in HPLC separation. HPLC-grade solvents and water that are commercially available are recommended for RP-HPLC. Dissolved gases present a problem in gradient systems where the solubility of the gas in a solvent may be less in a mixture than in a single solvent. Vacuum degassing and agitation by sonication are often used as useful degassing procedures. The solid particles in the solvents are drawn into the HPLC system; they can damage the sealing of pump valves or clog the top of the chromatographic column. Both pre- and post-pump filtration are also recommended.
Chromatographic Column—
The selection of a chromatographic column is empirically determined for each protein. Columns with 100
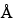
or 300
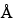
pore size and silica support can give optimal separation. For smaller peptides, column packings of octylsilane chemically bonded to totally porous silica articles 3 to 10 µm in diameter (L7) and of octadecylsilane chemically bonded to porous silica or ceramic microparticles 3 to 10 µm in diameter (L1) are more efficient than the butyl silane chemically bonded to totally porous silica particles 5 to 10 µm in diameter (L26).
Solvent—
The most commonly used solvent is water with acetonitrile as the organic modifier to which less than 0.1% of trifluoroacetic acid is added. If necessary, add isopropyl alcohol or n-propyl alcohol to solubilize the digest components, provided that the addition does not unduly increase the viscosity of the components.
Mobile Phase—
Buffered mobile phases containing phosphate are used to provide some flexibility in the selection of pH conditions, since shifts of pH in the 3.0 to 5.0 range enhance the separation of peptides containing acidic residues (e.g., glutamic and aspartic acids). Sodium or potassium phosphates, ammonium acetate, and phosphoric acid, with a pH between 2 and 7 (or higher for polymer-based supports), have also been used with acetonitrile gradients. Acetonitrile-containing trifluoroacetic acid is also used quite often.
Gradient Selection—
Gradients can be linear, nonlinear, or include step functions. A shallow gradient is recommended in order to separate complex mixtures. Gradients are optimized to provide clear resolution of one or two peaks that will become “marker” peaks for the test.
Isocratic Selection—
Isocratic HPLC systems using a single mobile phase are used on the basis of their convenience of use and improved detector responses. Optimal composition of a mobile phase to obtain clear resolution of each peak is sometimes difficult to establish. Mobile phases for which slight changes in component ratios or in pH significantly affect retention times of peaks in peptide maps should not be used in isocratic HPLC systems.
Other Parameters—
Temperature control of the column is usually necessary to achieve good reproducibility. The flow rates for the mobile phases range from 0.1 to 2.0 mL per minute, and the detection of peptides is performed with a UV detector at 200 to 230 nm. Other methods of detection have been used (e.g., postcolumn derivatization), but they are not as robust or as versatile as UV detection.
System Suitability—
The section
System Suitability under
Chromatography  621
621
provides an experimental means for measuring the overall performance of the test method. The acceptance criteria for system suitability depend on the identification of critical test parameters that affect data interpretation and acceptance. These critical parameters are also criteria that monitor peptide digestion and peptide analysis. An indicator that the desired digestion endpoint was achieved is the comparison with a reference standard or reference material, which is treated exactly as the article under test. The use of a USP Reference Standard in parallel with the protein under test is critical in the development and establishment of system suitability limits. In addition, a specimen chromatogram should be included with the USP Reference Standard or reference material for comparison purposes. Other indicators may include visual inspection of protein or peptide solubility, the absence of intact protein, or measurement of responses of a digestion-dependent peptide. The critical system suitability parameters for peptide analysis will depend on the particular mode of peptide separation and detection, and on the data analysis requirements.
When peptide mapping is used as an identification test, the system suitability requirements for the identified peptides cover selectivity and precision. In this case, as well as when identification of variant proteins is done, the identification of the primary structure of the peptide fragments in the peptide map provides both a verification of the known primary structure and the identification of protein variants by comparison with the peptide map of the USP Reference Standard or reference material for the specified protein. The use of a digested USP Reference Standard or reference material for a given protein in the determination of peptide resolution is the method of choice. For an analysis of a variant protein, a characterized mixture of a variant and a reference standard can be used, especially if the variant peptide is located in a less-resolved region of the map. The index of pattern consistency can be simply the number of major peptides detected. Peptide pattern consistency can be best defined by the resolution of peptide peaks. Chromatographic parameters—such as peak-to-peak resolution, maximum peak width, peak tailing factors, and column efficiency—may be used to define peptide resolution. Depending on the protein under test and the method of separation used, single peptide or multiple peptide resolution requirements may be necessary.
The replicate analysis of the digest of the USP Reference Standard or reference material for the protein under test yields measures of precision and quantitative recovery. Recovery of the identified peptides is generally ascertained by the use of internal or external peptide standards. The precision is expressed as the relative standard deviation (RSD). Differences in the recovery and precision of the identified peptides are expected; therefore, the system suitability limits will have to be established for both the recovery and the precision of the identified peptides. These limits are unique for a given protein and will be specified in the individual monograph.
Visual comparison of the relative retention times, the peak responses, the number of peaks, and the overall elution pattern is completed initially. It is then complemented and supported by mathematical analysis of the peak response ratios and by the chromatographic profile of a 1:1 (v/v) mixture of sample and USP Reference Standard or reference material digest. If all peaks in the sample digest and in the USP Reference Standard or reference material digest have the same relative retention times and peak response ratios, then the identity of the sample under test is confirmed.
If peaks that initially eluted with significantly different relative retention times are then observed as single peaks in the 1:1 mixture, the initial difference would be an indication of system variability. However, if separate peaks are observed in the 1:1 mixture, this would be evidence of the nonequivalence of the peptides in each peak. If a peak in the 1:1 mixture is significantly broader than the corresponding peak in the sample and USP Reference Standard or reference material digest, it may indicate the presence of different peptides. The use of computer-aided pattern recognition software for the analysis of peptide mapping data has been proposed and applied, but issues related to the validation of the computer software preclude its use in a compendial test in the near future. Other automated approaches have been used that employ mathematical formulas, models, and pattern recognition. Such approaches, for example, the automated identification of compounds by IR spectroscopy and the application of diode-array UV spectral analysis for identification of peptides, have been proposed. These methods have limitations due to inadequate resolutions, co-elution of fragments, or absolute peak response differences between USP Reference Standard or reference material and sample fragments.
The numerical comparison of the retention times and peak areas or peak heights can be done for a selected group of relevant peaks that have been correctly identified in the peptide maps. Peak areas can be calculated using one peak showing relatively small variation as an internal reference, keeping in mind that peak area integration is sensitive to baseline variation and likely to introduce error in the analysis. Alternatively, the percentage of each peptide peak height relative to the sum of all peak heights can be calculated for the sample under test. The percentage is then compared to that of the corresponding peak of the USP Reference Standard or reference material. The possibility of autohydrolysis of trypsin is monitored by producing a blank peptide map that is the peptide map obtained when a blank solution is treated with trypsin.
The minimum requirement for the qualification of peptide mapping is an approved test procedure that includes system suitability as a test control. In general, for an IND, qualification of peptide mapping for a protein is sufficient. As the regulatory approval process for the protein progresses, additional qualifications of the test can include a partial validation of the analytical procedure to provide assurance that the method will perform as intended in the development of a peptide map for the specified protein.
Analysis and Identification of Peptides
This section gives guidance on the use of peptide mapping during development in support of regulatory applications.
The use of a peptide map as a qualitative tool does not require the complete characterization of the individual peptide peaks. However, validation of peptide mapping in support of regulatory applications requires rigorous characterization of each of the individual peaks in the peptide map. Methods to characterize peaks range from N-terminal sequencing of each peak followed by amino acid analysis to the use of mass spectroscopy (MS).
For characterization purposes, when N-terminal sequencing and amino acid analysis are used, the analytical separation is scaled up. Because scale-up might affect the resolution of peptide peaks, it is necessary, using empirical data, to assure that there is no loss of resolution due to scale-up. Eluates corresponding to specific peptide peaks are collected, vacuum-concentrated, and chromatographed again, if necessary. Amino acid analysis of fragments may be limited by the peptide size. If the N-terminus is blocked, it may need to be cleared before sequencing. C-terminal sequencing of proteins or a combination of carboxypeptidase digestion and matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) MS can also be used for characterization purposes.
The use of MS for characterization of peptide fragments is by direct infusion of isolated peptides or by the use of on-line LC-MS for structure analysis. In general, it includes electrospray and MALDI-TOF analyzers as well as fast atom bombardment (FAB). Tandem MS has also been used to sequence a modified protein and to determine the type of amino acid modification that has occurred. The comparison of mass spectra of the digests before and after reduction provides a method to assign the disulfide bonds to the various sulfhydryl-containing peptides.
If regions of the primary structure are not clearly demonstrated by the peptide map, it might be necessary to develop a secondary peptide map. The goal of a validated method of characterization of a protein through peptide mapping is to reconcile and account for at least 95% of the theoretical composition of the protein structure.

