The temperature at which a substance passes from the liquid to the solid state upon cooling is a useful index to purity if heat is liberated when the solidification takes place, provided that any impurities present dissolve in the liquid only, and not in the solid. Pure substances have a well-defined freezing point, but mixtures generally freeze over a range of temperatures. For many mixtures, the congealing temperature, as determined by strict adherence to the following empirical methods, is a useful index of purity. The method for determining congealing temperatures set forth here is applicable to substances that melt between
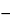
20

and 150

, the range of the thermometer used in the bath. The congealing temperature is the maximum point (or lacking a maximum, the point of inflection) in the temperature-time curve.
Apparatus—
Assemble an apparatus similar to that
illustrated,
Congealing Temperature Apparatus
in which the container for the substance is a 25- × 100-mm test tube. This is provided with a suitable, short-range thermometer suspended in the center, and a wire stirrer, about 30 cm long, bent at its lower end into a horizontal loop around the thermometer. Use a thermometer having a range not exceeding 30

, graduated in 0.1

divisions, and calibrated for, but not used at, 76-mm immersion. A suitable series of thermometers, covering a range from
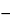
20

to +150

, is available as the ASTM E1 series 89C through 96C. Other temperature-measuring devices may be used if they are validated for this procedure (see
Thermometers  21
21
). Dimensions should be within ±20% of those given in the illustration.
The specimen container is supported, by means of a cork, in a suitable water-tight cylinder about 50 mm in internal diameter and 11 cm in length. The cylinder, in turn, is supported in a suitable bath sufficient to provide not less than a 37-mm layer surrounding the sides and bottom of the cylinder. The outside bath is provided with a suitable thermometer.
Procedure—
Melt the substance, if a solid, at a temperature not exceeding 20

above its expected congealing point, and pour it into the test tube to a height of 50 to 57 mm. Assemble the apparatus with the bulb of the test tube thermometer immersed halfway between the top and bottom of the specimen in the test tube. Fill the bath to about 12 mm from the top of the tube with suitable fluid at a temperature 4

to 5

below the expected congealing point.
In case the substance is a liquid at room temperature, carry out the determination using a bath temperature about 15

below the expected congealing point.
When the test specimen has cooled to about 5

above its expected congealing point, adjust the bath to a temperature 7

to 8

below the expected congealing point. Stir the specimen continuously during the remainder of the test by moving the loop up and down between the top and bottom of the specimen, at a regular rate of 20 complete cycles per minute.
Congelation frequently may be induced by rubbing the inner walls of the test tube with the thermometer, or by introducing a small fragment of the previously congealed substance. Pronounced supercooling may cause deviation from the normal pattern of temperature changes. If the latter occurs, repeat the test, introducing small particles of the material under test in solid form at 1

intervals as the temperature approaches the expected congealing point.
Record the reading of the test tube thermometer every 30 seconds. Continue stirring only so long as the temperature is gradually falling, stopping when the temperature becomes constant or starts to rise slightly. Continue recording the temperature in the test tube every 30 seconds for at least 3 minutes after the temperature again begins to fall after remaining constant.
The average of not less than four consecutive readings that lie within a range of 0.2

constitutes the congealing temperature. These readings lie about a point of inflection or a maximum, in the temperature-time curve, that occurs after the temperature becomes constant or starts to rise and before it again begins to fall. The average to the nearest 0.1

is the congealing temperature.

