OSMOLALITY
The osmolality of a solution
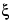 m
m is given by
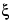 m
m =
S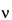 imiFm,i
imiFm,i.
The osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed in osmoles or milliosmoles per kilogram of solvent (Osmol per kg or mOsmol per kg, respectively), a unit that is similar to the molality of the solution. Thus, osmolality is a measure of the osmotic pressure exerted by a real solution across a semipermeable membrane. Like osmotic pressure, other colligative properties of a solution, such as vapor pressure lowering, boiling point elevation, and freezing point depression, are also directly related to the osmolality of the solution. Indeed, the osmolality of a solution is typically determined most accurately and conveniently by measuring freezing point depression (DTf):
DTf =
kf 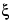 m
m
where
kf is the molal cryoscopic constant, which is a property of the solvent. For water, the value of
kf is 1.860

per Osmol. That is, 1 Osmol of a solute added to 1 kg of water lowers the freezing point by 1.860

.
OSMOLARITY
Osmolarity of a solution is a theoretical quantity expressed in osmoles per L (Osmol per L) of a solution and is widely used in clinical practice because it expresses osmoles as a function of volume. Osmolarity cannot be measured but is calculated theoretically from the experimentally measured value of osmolality.
Sometimes, osmolarity (
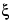 c
c) is calculated theoretically from the molar concentrations:
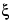 c
c =
S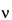 ici
ici
where
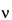 i
i is as defined above, and
ci is the molar concentration of the
ith solute in solution. For example, the osmolarity of a solution prepared by dissolving 1 g of vancomycin in 100 mL of 0.9% sodium chloride solution can be calculated as follows:
[3 × 10 g/L/1449.25(mol. wt. of vancomycin) + 2 × 9 g/L/58.44(mol. wt. of sodium chloride)] × 1000 = 329 mOsmol/L
The results suggest that the solution is slightly hyperosmotic because the osmolality of blood ranges between 285 and 310 mOsmol per kg. However, the solution is found to be hypo-osmotic and has an experimentally determined osmolality of 255 mOsmol per kg.
1 The example illustrates that osmolarity values calculated theoretically from the concentration of a solution should be interpreted cautiously and may not represent the osmotic properties of infusion solutions.
The discrepancy between theoretical (osmolarity) and experimental (osmolality) results is, in part, due to the fact that osmotic pressure is related to osmolality and not osmolarity. More significantly, the discrepancy between experimental results and the theoretical calculation is due to the fact that the osmotic pressure of a real solution is less than that of an ideal solution because of interactions between solute molecules or between solute and solvent molecules in a solution. Such interactions reduce the pressure exerted by solute molecules on a semipermeable membrane, reducing experimental values of osmolality compared to theoretical values. This difference is related to the molal osmotic coefficient (Fm,i). The example also illustrates the importance of determining the osmolality of a solution experimentally, rather than calculating the value theoretically.
MEASUREMENT OF OSMOLALITY
The osmolality of a solution is commonly determined by the measurement of the freezing point depression of the solution.
Apparatus—
The apparatus, an osmometer for freezing point depression measurement, consists of the following: a means of cooling the container used for the measurement; a resistor sensitive to temperature (thermistor), with an appropriate current- or potential-difference measurement device that may be graduated in temperature change or in osmolality; and a means of mixing the sample.
Osmometers that measure the vapor pressures of solutions are less frequently employed. They require a smaller volume of specimen (generally about 5 µL), but the accuracy and precision of the resulting osmolality determination are comparable to those obtained by the use of osmometers that depend upon the observed freezing points of solutions.
Standard Solutions—
Prepare
Standard Solutions as specified in
Table 1, as necessary.
Table 1. Standard Solutions for Osmometer Calibration2
Standard Solutions
(Weight in g of sodium chloride per kg of water) |
Osmolality
(mOsmol/kg)
(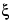 m) m) |
Molal Osmotic
Coefficient
(Fm, NaCl) |
Freezing Point
Depression ( ) )
DTf |
 |
 |
 |
 |
| 3.087 |
100 |
0.9463 |
0.186 |
| 6.260 |
200 |
0.9337 |
0.372 |
| 9.463 |
300 |
0.9264 |
0.558 |
| 12.684 |
400 |
0.9215 |
0.744 |
| 15.916 |
500 |
0.9180 |
0.930 |
| 19.147 |
600 |
0.9157 |
1.116 |
| 22.380 |
700 |
0.9140 |
1.302 |
|
2
Adapted from the European Pharmacopoeia, 4th Edition, 2002, p. 50.
|
Test Solution—
For a solid for injection, constitute with the appropriate diluent as specified in the instructions on the labeling. For solutions, use the sample as is. [note—A solution can be diluted to bring it within the range of measurement of the osmometer, if necessary, but the results must be expressed as that of the diluted solution and must NOT be multiplied by a dilution factor to calculate the osmolality of the original solution, unless otherwise indicated in the monograph. The molal osmotic coefficient is a function of concentration. Therefore, it changes with dilution.]
Procedure—
First, calibrate the instrument by the manufacturer's instructions. Confirm the instrument calibration with at least two solutions from
Table 1 such that the osmolalities of the
Standard Solutions span the expected range of osmolality of the
Test Solution. The instrument reading should be within ±2 mOsmol/kg from the
Standard Solution (over the standard range of 100 to 700 mOsmol/kg). Introduce an appropriate volume of each
Standard Solution into the measurement cell as per the manufacturer's instructions, and start the cooling system. Usually, the mixing device is programmed to operate at a temperature below the lowest temperature expected from the freezing point depression. The apparatus indicates when the equilibrium is attained. Calibrate the osmometer using an appropriate adjustment device such that the reading corresponds to either the osmolality or freezing point depression value of the
Standard Solution shown in
Table 1. [note—Some instruments indicate osmolality and some others show freezing point depression.
] Before each measurement, rinse the measurement cell at least twice with the solution to be tested. Repeat the procedure with each
Test Solution. Read the osmolality of the
Test Solution directly, or calculate it from the measured freezing point depression.
Assuming that the value of the osmotic coefficient is essentially the same whether the concentration is expressed in molality or molarity, the experimentally determined osmolality of a solution can be converted to osmolarity in the same manner in which the concentration of a solution is converted from molality to molarity. Unless a solution is very concentrated, the osmolarity of a solution (
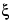 c
c) can be calculated from its experimentally determined osmolality (
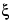 m
m):
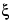 c
c = 1000
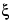 m
m / (1000 /

+
Swi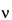 i
i)
where
wi is the weight in g; and
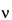 i
i is the partial specific volume, in mL per g, of the
ith solute. The partial specific volume of a solute is the change in volume of a solution when an additional 1 g of solute is dissolved in the solution. This volume can be determined by the measurement of densities of the solution before and after the addition of the solute. The partial specific volumes of salts are generally very small, around 0.1 mL per g. However, those of other solutes are generally higher. For example, the partial specific volumes of amino acids are in the range of 0.6–0.9 mL per g. It can be shown from the above equation correlating osmolarity with osmolality that,
where

is the density of the solution, and
c is the total solute concentration, both expressed in g per mL. Thus, alternatively, the osmolarity can also be calculated from experimentally determined osmolality from the measurement of density of the solution by a suitable method and the total weight of the solute, after correction for water content, dissolved per mL of the solution.

