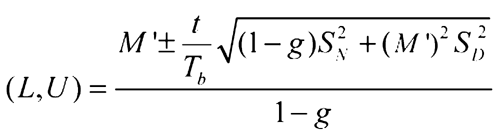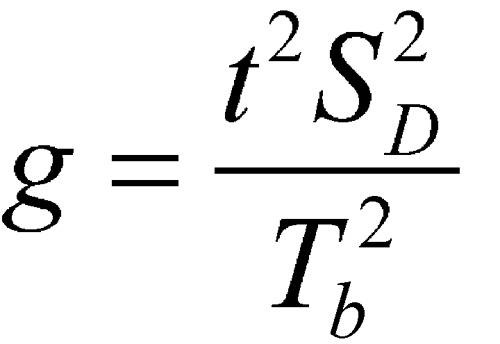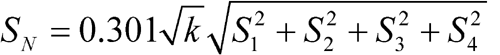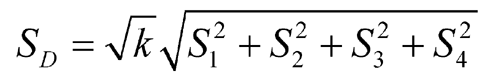The most prominent manifestation of insulin activity, an abrupt decrease in blood glucose, was the basis for biologic assay from the time of its first clinical use. The procedure, although relatively cumbersome, has the great merit of accurately reflecting the effect on the diabetic patient. The advent of practical yet sophisticated physicochemical methods (e.g., liquid chromatography) to measure insulin potency quantitatively has resulted in a more accurate and precise compendial test for insulin and insulin products. However, the bioidentity of insulin and insulin products cannot be assessed by these methods. Thus, a qualitative test in rabbits is included in this chapter, and its use is called for in the appropriate monographs.
The Rabbit Blood Sugar Method—Quantitative is used to determine the potency of Insulin Reference Standards, for the validation of the stability of new insulin preparations, and to determine the specific activities of insulin analogs.
RABBIT BLOOD SUGAR METHOD—QUANTITATIVE
USP Reference Standards  11
11 —
USP Dextrose RS. USP Insulin RS. USP Insulin (Beef) RS. USP Insulin Human RS. USP Insulin (Pork) RS.
—
USP Dextrose RS. USP Insulin RS. USP Insulin (Beef) RS. USP Insulin Human RS. USP Insulin (Pork) RS.
Diluent—
Prepare an aqueous solution containing 0.1% to 0.25% (w/v) of either cresol or phenol, 1.4% to 1.8% (w/v) of glycerin, and sufficient hydrochloric acid to produce a pH between 2.5 and 3.5, unless otherwise directed in the individual monograph.
Standard Stock Solution—
Dissolve either a suitable quantity of accurately weighed USP Insulin RS or a vial of lyophilized USP Insulin RS of the appropriate species in Diluent to make a Standard Stock Solution containing 40 USP Insulin Units per mL and having a pH between 2.5 and 3.5, unless otherwise directed in the individual monograph. Store in a cold place, protected from freezing, and use within 6 months.
Standard Solutions—
Dilute portions of the Standard Stock Solution with Diluent to make two solutions, one to contain 1.0 USP Insulin Unit per mL (Standard Solution 1), and the other to contain 2.0 USP Insulin Units per mL (Standard Solution 2).
Assay Stock Solution—
Proceed as directed under Standard Stock Solution, except to use a suitable quantity of the preparation under test in place of USP Insulin RS. The Assay Stock Solution contains about 40 USP Insulin Units per mL.
Assay Solutions—
Dilute portions of the Assay Stock Solution with Diluent to make two dilutions of the preparation under test, one of which may be expected, on the basis of the assumed potency, to contain 1.0 USP Insulin Unit per mL (Assay Solution 1), and the other to contain 2.0 USP Insulin Units per mL (Assay Solution 2). In the case of neutral insulin injection, adjust to a pH of 2.5 to 3.5 prior to making the dilutions.
Doses of the Solutions To Be Injected—
Select on the basis of trial or experience the dose of the dilutions to be injected, the volume of which usually will be between 0.30 mL and 0.50 mL. For each animal the volume of the Standard Solution is the same as that of the Assay Solution.
Preparation of Animal—
Select suitable, healthy rabbits each weighing not less than 1.8 kg. Keep the rabbits in the laboratory for not less than 1 week before use in the assay, maintaining them on an adequate uniform diet, with water available at all times.
Procedure—
Divide the rabbits into four equal groups of preferably not less than six rabbits each. On the preceding day, approximately 20 hours before the assay, provide each rabbit with an amount of food that will be consumed within 6 hours. Follow the same feeding schedule before each test day. During the assay, withhold all food until after the final blood specimen is taken. Handle the rabbits with care in order to avoid undue excitement, and inject subcutaneously the doses indicated in the following design (see Table 1), the second injection being made on the day after the first injection, or not more than 1 week later. The time between the first and second injection is the same for all rabbits.
Table 1
| Group | ||
| 1 | Standard Solution 2 | Assay Solution 1 |
| 2 | Standard Solution 1 | Assay Solution 2 |
| 3 | Assay Solution 2 | Standard Solution 1 |
| 4 | Assay Solution 1 | Standard Solution 2 |
Blood Samples—
At 1 hour ± 5 minutes and 2½ hours ± 5 minutes after the time of injection, obtain from each rabbit a suitable blood specimen from a marginal ear vein. Blood can also be collected effectively from the central auricular artery.
Dextrose Determination—
Determine the dextrose content of the blood specimens by a suitable procedure that is adapted to automated analysis. The following procedure may be used.
Anticoagulant Solution—
Dissolve 1 g of edetate sodium and 200 mg of sodium fluoride in 1 L of water, and mix.
Dextrose Standard Preparations—
Transfer known concentrations of USP Dextrose RS to suitable vessels, and dilute quantitatively and stepwise with Anticoagulant Solution (1:9) to obtain a range of Dextrose Standard Preparations containing between 20 and 100 mg per 100 mL, having known concentrations similar to the concentrations in the rabbit blood samples.
Test Preparations—
Pipet into separate, suitable vessels 0.1 mL of each Blood Sample and 0.9 mL of Anticoagulant Solution.
Procedure—
Subject the Test Preparations to dialysis across a semipermeable membrane for a sufficient time so that the dextrose passes through the membrane into a saline TS solution containing glucose oxidase, horseradish peroxidase, 3-methyl-2-benzothiazolinone hydrazone hydrochloride TS, and N,N-dimethylaniline. The absorbances of the Test Preparations are determined at 600 nm in a recording colorimeter. The absorbances of the Dextrose Standard Preparations are similarly determined at the start and the end of each run.
Calculation—
Calculate the response of each rabbit to each injection from the sum of the two blood-sugar values, and subtract its response, disregarding the chronological order in which the responses were observed, to obtain the individual differences, y, as shown in Table 2.
When the data for one or more rabbits are missing in an assay, do not use the confidence interval formulas given here, but seek statistical help. The data can still be analyzed with proper analysis of variance.
When the number of rabbits, f, carried through the assay is the same in each group, total the y's in each group and compute Ta = –T1 + T2 + T3 – T4 and Tb = T1 + T2 + T3 + T4. The logarithm of the relative potency of the test dilutions is M ¢ = 0.301Ta / Tb. The potency of the injection in USP Units per mg equals the antilog (log R + M ¢), where R = vS / vU, in which vS is the number of USP Units per mL of the Standard solution and vU is the number of mg of insulin per mL of the corresponding Assay solution.
Determine the 95% confidence interval for the log-relative potency using Fieller's Theorem (see Appendix and Design and Analysis of Biological Assays  111
111 ). If the confidence interval is more than 0.082, which corresponds at P = 0.95 to confidence limits of about ±10% of the computed potency, repeat the assay until the combined data of the two or more assays, redetermined as described in Combination of Independent Assays under Design and Analysis of Biological Assays
). If the confidence interval is more than 0.082, which corresponds at P = 0.95 to confidence limits of about ±10% of the computed potency, repeat the assay until the combined data of the two or more assays, redetermined as described in Combination of Independent Assays under Design and Analysis of Biological Assays  111
111 , meet this acceptable limit.
, meet this acceptable limit.
Table 2
| Group | Differences | Individual Response (y) |
Total Response (T) |
Standard Deviations of Differences (S) |
| 1 | Standard Solution 2 – Assay Solution 1 | y1 | T1 | S1 |
| 2 | Assay Solution 2 – Standard Solution 1 | y2 | T2 | S2 |
| 3 | Assay Solution 2 – Standard Solution 1 | y3 | T3 | S3 |
| 4 | Standard Solution 2 – Assay Solution 1 | y4 | T4 | S4 |
BIOIDENTITY TEST
Proceed as directed for Rabbit Blood Sugar Method—Quantitative with the following modifications:
Procedure—
Divide the rabbits into four equal groups of two rabbits each.
Calculation—
Proceed as directed for Calculation under Rabbit Blood Sugar Method—Quantitative, but do not determine the confidence interval of the log-relative potency, M¢.
Interpretation—
If the potency value obtained is not less than 15 USP Units per mg, the Bioidentity Test requirement is met. If the potency value is less than 15 USP Units per mg, repeat the test using eight more rabbits. If the average potency of the two sets of tests is not less than 15 USP Units per mg, the requirement of the test is met.
Appendix—Fieller's Theorem for Determining the Confidence Interval for a Ratio
This version of Fieller's Theorem is for the case where the numerator and denominator are uncorrelated. The equation assumes the numerator and denominator are normally distributed and the groups of rabbits are equal-sized.
Then, the 95% confidence interval for the ratio is:
where f (degrees of freedom in the standard errors) = 4(k – 1), where k is the number of rabbits in a group, t is the upper 97.5 percentile of the t-distribution with f degrees of freedom, and
If g 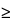 1, the denominator is not significantly different from 0 and the formula does not work.
1, the denominator is not significantly different from 0 and the formula does not work.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Larry N. Callahan, Ph.D.
Senior Scientist 1-301-816-8385 |
(BBPP05) Biologics and Biotechnology - Proteins and Polysaccharides |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 116
Pharmacopeial Forum: Volume No. 30(5) Page 1675