This general information chapter has been harmonized with the corresponding texts of the European Pharmacopoeia and the Japanese Pharmacopoeia. The harmonized texts of these three pharmacopeias are therefore interchangeable, and the methods of the European Pharmacopoeia and/or the Japanese Pharmacopoeia may be used for demonstration of compliance instead of the present United States Pharmacopeia general information chapter method. These pharmacopeias have undertaken not to make any unilateral change to this harmonized chapter.
This chapter provides guidelines for the friability determination of compressed, uncoated tablets. The test procedure presented in this chapter is generally applicable to most compressed tablets. Measurement of tablet friability supplements other physical strength measurements, such as tablet breaking force.
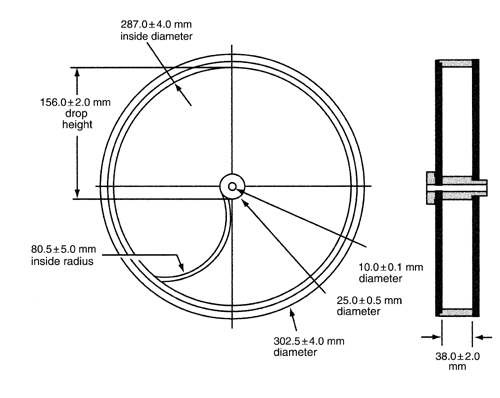
Use a drum,* with an internal diameter between 283 and 291 mm and a depth between 36 and 40 mm, of transparent synthetic polymer with polished internal surfaces, and subject to minimum static build-up (see figure for a typical apparatus). One side of the drum is removable. The tablets are tumbled at each turn of the drum by a curved projection with an inside radius between 75.5 and 85.5 mm that extends from the middle of the drum to the outer wall. The outer diameter of the central ring is between 24.5 and 25.5 mm. The drum is attached to the horizontal axis of a device that rotates at 25 ±1 rpm. Thus, at each turn the tablets roll or slide and fall onto the drum wall or onto each other.
For tablets with a unit weight equal to or less than 650 mg, take a sample of whole tablets corresponding as near as possible to 6.5 g. For tablets with a unit weight of more than 650 mg, take a sample of 10 whole tablets. The tablets should be carefully dedusted prior to testing. Accurately weigh the tablet sample, and place the tablets in the drum. Rotate the drum 100 times, and remove the tablets. Remove any loose dust from the tablets as before, and accurately weigh.
Generally, the test is run once. If obviously cracked, cleaved, or broken tablets are present in the tablet sample after tumbling, the sample fails the test. If the results are difficult to interpret or if the weight loss is greater than the targeted value, the test should be repeated twice and the mean of the three tests determined. A maximum mean weight loss from the three samples of not more than 1.0% is considered acceptable for most products.
If tablet size or shape causes irregular tumbling, adjust the drum base so that the base forms an angle of about 10 with the horizontal and the tablets no longer bind together when lying next to each other, which prevents them from falling freely.
with the horizontal and the tablets no longer bind together when lying next to each other, which prevents them from falling freely.
Effervescent tablets and chewable tablets may have different specifications as far as friability is concerned. In the case of hygroscopic tablets, an appropriate humidity-controlled environment is required for testing.
Drums with dual scooping projections, or an apparatus with more than one drum, for the running of multiple samples at one time, are also permitted.
*
The apparatus meeting these specifications is available from laboratory supply houses such as VanKel Technology Group, 13000 Weston Parkway, Cary, NC 27513, or from Erweka Instruments, Inc., 56 Quirk Road, Milford, CT 06460.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | William E. Brown
Senior Scientist 1-301-816-8380 |
(PDF05) Pharmaceutical Dosage Forms 05 |
USP32–NF27 Page 725
Pharmacopeial Forum: Volume No. 31(6) Page 1735