The requirements apply to sterile and nonpyrogenic assemblies or devices in contact directly or indirectly with the cardiovascular system, the lymphatic system, or cerebrospinal fluid. This includes, but is not limited to, solution administration sets, extension sets, transfer sets, blood administration sets, intravenous catheters, implants extracorporeal oxygenator tubings and accessories, dialysers and dialysis tubing and accessories, heart valves, vascular grafts, intramuscular drug delivery catheters, and transfusion and infusion assemblies. These requirements do not apply to orthopedic products, latex gloves, or wound dressings.
Bacterial Endotoxins— Proceed as directed under Bacterial Endotoxins Test
For medical devices, the endotoxin limit is not more than 20.0 USP Endotoxin Units per device except that for those medical devices in contact with the cerebrospinal fluid the limit is not more than 2.15 USP Endotoxin Units per device.
A device that fails this test can be retested once by another Bacterial Endotoxins test. For devices that cannot be tested by the Bacterial Endotoxins Test  85
85 because of nonremovable inhibition or enhancement, the Pyrogen Test
because of nonremovable inhibition or enhancement, the Pyrogen Test  151
151 is applied.
is applied.
Preparation of Devices—
Select not less than 3 and not more than 10 devices. Rinse or soak the devices with LAL Reagent Water. The volume of rinsing or extracting solution may be adjusted for the size and configuration of the device.
For devices labeled “nonpyrogenic fluid pathway,” flush the fluid pathway with extracting fluid that has been heated to 37 ± 1.0 , keeping the extracting fluid in contact with the relevant pathway for not less than 1 hour at controlled room temperature. Extracts may be combined, where appropriate. The endotoxin limit for the rinsing or extracting solution is calculated by the formula:
, keeping the extracting fluid in contact with the relevant pathway for not less than 1 hour at controlled room temperature. Extracts may be combined, where appropriate. The endotoxin limit for the rinsing or extracting solution is calculated by the formula:
(K × N) / (V)
where K is equal to the amount of endotoxin allowed per device, N is equal to the number of devices tested, and V is equal to the total volume of the extract or rinse. If the undiluted rinsing or extracting solution is unsuitable for the Bacterial Endotoxins Test  85
85 , repeat the inhibition or enhancement test after neutralization and removal of the interfering substances or after the solution has been diluted by a factor not exceeding the Maximum Valid Dilution. The Maximum Valid Dilution for devices is calculated by dividing the endotoxin limit by the labeled sensitivity
, repeat the inhibition or enhancement test after neutralization and removal of the interfering substances or after the solution has been diluted by a factor not exceeding the Maximum Valid Dilution. The Maximum Valid Dilution for devices is calculated by dividing the endotoxin limit by the labeled sensitivity 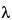 of the LAL reagent used.
of the LAL reagent used.
Pyrogen— For samples that cannot be tested by the Bacterial Endotoxins Test because of nonremovable inhibition or enhancement of the test, the Pyrogen Test
Other Requirements— The portions of medical devices that are made of plastics or other polymers meet the requirements specified for Biological Tests—Plastics and Other Polymers under Containers—Plastics
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(GTMDB05) General Toxicology and Medical Device Biocompatibility |
USP32–NF27 Page 125