The following procedures are applicable for determining whether a Pharmacopeial article purporting to be sterile complies with the requirements set forth in the individual monograph with respect to the test for sterility. Pharmacopeial articles are to be tested by the Membrane Filtration method under Test for Sterility of the Product to be Examined where the nature of the product permits. If the membrane filtration technique is unsuitable, use the Direct Inoculation of the Culture Medium method under Test for Sterility of the Product to be Examined. All devices, with the exception of Devices with Pathways Labeled Sterile, are tested using the Direct Inoculation of the Culture Medium method. Provisions for retesting are included under Observation and Interpretation of Results.
Because sterility testing is a very exacting procedure, where asepsis of the procedure must be ensured for a correct interpretation of results, it is important that personnel be properly trained and qualified. The test for sterility is carried out under aseptic conditions. In order to achieve such conditions, the test environment has to be adapted to the way in which the sterility test is performed. The precautions taken to avoid contamination are such that they do not affect any microorganisms that are to be revealed in the test. The working conditions in which the tests are performed are monitored regularly by appropriate sampling of the working area and by carrying out appropriate controls.
These Pharmacopeial procedures are not by themselves designed to ensure that a batch of product is sterile or has been sterilized. This is accomplished primarily by validation of the sterilization process or of the aseptic processing procedures.
When evidence of microbial contamination in the article is obtained by the appropriate Pharmacopeial method, the result so obtained is conclusive evidence of failure of the article to meet the requirements of the test for sterility, even if a different result is obtained by an alternative procedure.  For additional information on sterility testing, see Sterilization and Sterility Assurance of Compendial Articles
For additional information on sterility testing, see Sterilization and Sterility Assurance of Compendial Articles  1211
1211 .
.
MEDIA
Prepare media for the tests as described below, or dehydrated formulations may be used provided that, when reconstituted as directed by the manufacturer or distributor, they meet the requirements of the Growth Promotion Test of Aerobes, Anaerobes, and Fungi. Media are sterilized using a validated process.
The following culture media have been found to be suitable for the test for sterility. Fluid Thioglycollate Medium is primarily intended for the culture of anaerobic bacteria. However, it will also detect aerobic bacteria. Soybean–Casein Digest Medium is suitable for the culture of both fungi and aerobic bacteria.
Fluid Thioglycollate Medium
| l-Cystine | 0.5 g |
| Sodium Chloride | 2.5 g |
| Dextrose (C6H12O6·H2O) | 5.5/5.0 g |
| Agar, granulated (moisture content not exceeding 15%) |
0.75 g |
| Yeast Extract (water-soluble) | 5.0 g |
| Pancreatic Digest of Casein | 15.0 g |
| Sodium Thioglycollate | 0.5 g |
| 0.3 mL | |
| Resazurin Sodium Solution (1 in 1000), freshly prepared |
1.0 mL |
| Purified Water | 1000 mL |
Mix the l-cystine, sodium chloride, dextrose, yeast extract, and pancreatic digest of casein with the purified water, and heat until solution is effected. Dissolve the sodium thioglycollate or thioglycolic acid in the solution and, if necessary, add 1 N sodium hydroxide so that, after sterilization, the solution will have a pH of 7.1 ± 0.2. If filtration is necessary, heat the solution again without boiling, and filter while hot through moistened filter paper. Add the resazurin sodium solution, mix, and place the medium in suitable vessels that provide a ratio of surface to depth of medium such that not more than the upper half of the medium has undergone a color change indicative of oxygen uptake at the end of the incubation period. Sterilize using a validated process. If the medium is stored, store at a temperature between 2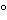 and 25
and 25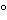 in a sterile, airtight container. If more than the upper one-third of the medium has acquired a pink color, the medium may be restored once by heating the containers in a water-bath or in free-flowing steam until the pink color disappears and by cooling quickly, taking care to prevent the introduction of nonsterile air into the container.
in a sterile, airtight container. If more than the upper one-third of the medium has acquired a pink color, the medium may be restored once by heating the containers in a water-bath or in free-flowing steam until the pink color disappears and by cooling quickly, taking care to prevent the introduction of nonsterile air into the container.
Fluid Thioglycollate Medium is to be incubated at 32.5 ± 2.5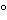 .
.
Prepare a mixture having the same composition as that of the Fluid Thioglycollate Medium, but omitting the agar and the resazurin sodium solution, sterilize as directed above, and allow to cool prior to use. The pH after sterilization is 7.1 ± 0.2. Incubate under anaerobic conditions for the duration of the incubation period.
Alternative Fluid Thioglycollate Medium is to be incubated at 32.5 ± 2.5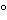 .
.
Soybean–Casein Digest Medium
| Pancreatic Digest of Casein | 17.0 g |
| Papaic Digest of Soybean Meal | 3.0 g |
| Sodium Chloride | 5.0 g |
| Dibasic Potassium Phosphate | 2.5 g |
| Dextrose (C6H12O6·H2O) | 2.5/2.3 g |
| Purified Water | 1000 mL |
Dissolve the solids in the Purified Water, heating slightly to effect a solution. Cool the solution to room temperature, and adjust the pH with 1 N sodium hydroxide so that, after sterilization, it will have a pH of 7.3 ± 0.2. Filter, if necessary to clarify, dispense into suitable containers, and sterilize using a validated procedure. Store at a temperature between 2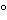 and 25
and 25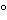 in a sterile well-closed container, unless it is intended for immediate use.
in a sterile well-closed container, unless it is intended for immediate use.
Soybean–Casein Digest Medium is to be incubated at 22.5 ± 2.5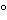 .
.
Where sterility test media are to be used in the Direct Inoculation of the Culture Medium method under Test for Sterility of the Product to be Examined, modify the preparation of Fluid Thioglycollate Medium and the Soybean–Casein Digest Medium as follows. To the containers of each medium, transfer aseptically a quantity of 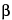 -lactamase sufficient to inactivate the amount of antibiotic in the specimen under test. Determine the quantity of
-lactamase sufficient to inactivate the amount of antibiotic in the specimen under test. Determine the quantity of 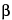 -lactamase required to inactivate the antibiotic by using a
-lactamase required to inactivate the antibiotic by using a 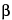 -lactamase preparation that has been assayed previously for its penicillin- or cephalosporin-inactivating power. [note—Supplemented
-lactamase preparation that has been assayed previously for its penicillin- or cephalosporin-inactivating power. [note—Supplemented 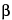 -lactamase media can also be used in the membrane filtration test.]
-lactamase media can also be used in the membrane filtration test.]
Alternatively (in an area completely separate from that used for sterility testing), confirm that an appropriate amount of 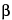 -lactamase is incorporated into the medium, following either method under Validation Test, using less than 100 colony-forming units (cfu) of Staphylococcus aureus (see Table 1) as the challenge. Typical microbial growth of the inoculated culture must be observed as a confirmation that the
-lactamase is incorporated into the medium, following either method under Validation Test, using less than 100 colony-forming units (cfu) of Staphylococcus aureus (see Table 1) as the challenge. Typical microbial growth of the inoculated culture must be observed as a confirmation that the 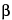 -lactamase concentration is appropriate.
-lactamase concentration is appropriate.
Table 1. Strains of the Test Microorganisms Suitable for Use in the Growth Promotion Test and the Validation Test
| Aerobic bacteria | ||
| Staphylococcus aureus |
ATCC 6538, CIP 4.83, NCTC 10788, NCIMB 9518 | |
| Bacillus subtilis | ATCC 6633, CIP 52.62, NCIMB 8054 | |
| Pseudomonas aeruginosa |
ATCC 9027, NCIMB 8626, CIP 82.118 | |
| Anaerobic bacterium | ||
| Clostridium sporogenes |
ATCC 19404, CIP 79.3, NCTC 532 or ATCC 11437 | |
| Fungi | ||
| Candida albicans | ATCC 10231, IP 48.72, NCPF 3179 | |
| Aspergillus niger | ATCC 16404, IP 1431.83, IMI 149007 | |
|
|
||
|
|
||
|
|
||
Suitability Tests
The media used comply with the following tests, carried out before, or in parallel, with the test on the product to be examined.
sterility
Confirm the sterility of each sterilized batch of medium by incubating a portion of the media at the specified incubation temperature for 14 days. No growth of microorganisms occurs.
growth promotion test of aerobes, anaerobes, and fungi
Inoculate portions of Fluid Thioglycollate Medium with a small number (not more than 100 cfu) of the following microorganisms, using a separate portion of medium for each of the following species of microorganism: Clostridium sporogenes, Pseudomonas aeruginosa, and Staphylococcus aureus.  Inoculate portions of Alternative Fluid Thioglycollate Medium with a small number (not more than 100 cfu) of Clostridium sporogenes.
Inoculate portions of Alternative Fluid Thioglycollate Medium with a small number (not more than 100 cfu) of Clostridium sporogenes. Inoculate portions of Soybean–Casein Digest Medium with a small number (not more than 100 cfu) of the following microorganisms, using a separate portion of medium for each of the following species of microorganism: Aspergillus niger, Bacillus subtilis, and Candida albicans. Incubate for not more than 3 days in the case of bacteria and not more than 5 days in the case of fungi.
Inoculate portions of Soybean–Casein Digest Medium with a small number (not more than 100 cfu) of the following microorganisms, using a separate portion of medium for each of the following species of microorganism: Aspergillus niger, Bacillus subtilis, and Candida albicans. Incubate for not more than 3 days in the case of bacteria and not more than 5 days in the case of fungi.
The media are suitable if a clearly visible growth of the microorganisms occurs.
If prepared media are stored in unsealed containers, they can be used for 1 month, provided that they are tested for growth promotion within 2 weeks of the time of use and that color indicator requirements are met. If stored in tight containers, the media can be used for 1 year, provided that they are tested for growth promotion within 3 months of the time of use and that the color indicator requirements are met.
Fluid A
preparation
Dissolve 1 g of peptic digest of animal tissue in water to make 1 L, filter or centrifuge to clarify, if necessary, and adjust to a pH of 7.1 ± 0.2. Dispense into containers, and sterilize using a validated process.
preparation for penicillins or cephalosporins
Aseptically add to the above Preparation, if necessary, a quantity of sterile 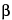 -lactamase sufficient to inactivate any residual antibiotic activity on the membranes after the solution of the test specimen has been filtered (see Media for Penicillins or Cephalosporins).
-lactamase sufficient to inactivate any residual antibiotic activity on the membranes after the solution of the test specimen has been filtered (see Media for Penicillins or Cephalosporins).
Fluid D
To each L of Fluid A add 1 mL of polysorbate 80, adjust to a pH of 7.1 ± 0.2, dispense into containers, and sterilize using a validated process. Use this fluid for articles containing lecithin or oil, or for devices labeled as “sterile pathway.”
Fluid K
Dissolve 5.0 g of peptic digest of animal tissue, 3.0 g of beef extract, and 10.0 g of polysorbate 80 in water to make 1 L. Adjust the pH to obtain, after sterilization, a pH of 6.9 ± 0.2. Dispense into containers, and sterilize using a validated process.
VALIDATION TEST
Carry out a test as described below under Test for Sterility of the Product to be Examined using exactly the same methods, except for the following modifications.
Membrane Filtration
After transferring the content of the container or containers to be tested to the membrane, add an inoculum of a small number of viable microorganisms (not more than 100 cfu) to the final portion of sterile diluent used to rinse the filter.
Direct Inoculation
After transferring the contents of the container or containers to be tested (for catgut and other surgical sutures for veterinary use: strands) to the culture medium, add an inoculum of a small number of viable microorganisms (not more than 100 cfu) to the medium.
In both cases use the same microorganisms as those described above under Growth Promotion Test of Aerobes, Anaerobes, and Fungi. Perform a growth promotion test as a positive control. Incubate all the containers containing medium for not more than 5 days.
If clearly visible growth of microorganisms is obtained after the incubation, visually comparable to that in the control vessel without product, either the product possesses no antimicrobial activity under the conditions of the test or such activity has been satisfactorily eliminated. The test for sterility may then be carried out without further modification.
If clearly visible growth is not obtained in the presence of the product to be tested, visually comparable to that in the control vessels without product, the product possesses antimicrobial activity that has not been satisfactorily eliminated under the conditions of the test. Modify the conditions in order to eliminate the antimicrobial activity, and repeat the validation test.
This validation is performed (a) when the test for sterility has to be carried out on a new product; and (b) whenever there is a change in the experimental conditions of the test. The validation may be performed simultaneously with the Test for Sterility of the Product to be Examined.
TEST FOR STERILITY OF THE PRODUCT TO BE EXAMINED
Unless otherwise specified elsewhere in this chapter or in the individual monograph, test the number of articles specified in Table 3. If the contents of each article are of sufficient quantity (see Table 2), they may be divided so that equal appropriate portions are added to each of the specified media. [note—Perform sterility testing employing two or more of the specified media.] If each article does not contain sufficient quantities for each medium, use twice the number of articles indicated in Table 3.
Table 2. Minimum Quantity to be Used for Each Medium
| Quantity per Container | Minimum Quantity to be Used (unless otherwise justified and authorized) |
| Liquids (other than antibiotics) | |
| Less than 1 mL | The whole contents of each container |
| 1–40 mL | Half the contents of each container, but not less than 1 mL |
| Greater than 40 mL, and not greater than 100 mL | 20 mL |
| Greater than 100 mL | 10% of the contents of the container, but not less than 20 mL |
| Antibiotic liquids | 1 mL |
| Other preparations soluble in water or in isopropyl myristate | The whole contents of each container to provide not less than 200 mg |
| Insoluble preparations, creams, and ointments to be suspended or emulsified | Use the contents of each container to provide not less than 200 mg |
| Solids | |
| Less than 50 mg | The whole contents of each container |
| 50 mg or more, but less than 300 mg | Half the contents of each container, but not less than 50 mg |
| 300 mg–5 g | 150 mg |
| Greater than 5 g | 500 mg |
| Devices | |
| Catgut and other surgical sutures for veterinary use | 3 sections of a strand (each 30-cm long) |
| |
100 mg per package |
| Sutures and other individually packaged single-use material | The whole device |
| Other medical devices | The whole device, cut into pieces or disassembled |
Table 3. Minimum Number of Articles to be Tested in Relation to the Number of Articles in the Batch
| Number of Items in the Batch | Minimum Number of Items to be Tested for Each Medium (unless otherwise justified and authorized)* |
| Parenteral preparations | |
| Not more than 100 containers | 10% or 4 containers, whichever is the greater |
| More than 100 but not more than 500 containers | 10 containers |
| More than 500 containers | 2% or 20 containers, whichever is less |
| 2% or 10 containers, whichever is less | |
| Antibiotic solids | |
| Pharmacy bulk packages (<5 g) | 20 containers |
| Pharmacy bulk packages ( |
6 containers |
| Bulks and blends | See Bulk solid products |
| Ophthalmic and other noninjectable preparations | |
| Not more than 200 containers | 5% or 2 containers, whichever is the greater |
| More than 200 containers | 10 containers |
| If the product is presented in the form of single-dose containers, apply the scheme shown above for preparations for parenteral use. |
|
| Devices | |
| Catgut and other surgical sutures for veterinary use | 2% or 5 packages, whichever is the greater, up to a maximum total of 20 packages |
| 10% or 4 articles, whichever is greater | |
| More than 100, but not more than 500 articles | 10 articles |
| More than 500 articles | 2% or 20 articles, whichever is less |
| Bulk solid products | |
| Up to 4 containers | Each container |
| More than 4 containers, but not more than 50 containers | 20% or 4 containers, whichever is greater |
| More than 50 containers | 2% or 10 containers, whichever is greater |
|
*
If the contents of one container are enough to inoculate the two media, this column gives the number of containers needed for both the media together.
|
|
The test may be carried out using the technique of Membrane Filtration or by Direct Inoculation of the Culture Medium with the product to be examined. Appropriate negative controls are included. The technique of membrane filtration is used whenever the nature of the product permits; that is, for filterable aqueous preparations, for alcoholic or oily preparations, and for preparations miscible with, or soluble in, aqueous or oily solvents, provided these solvents do not have an antimicrobial effect in the conditions of the test.
Membrane Filtration
Use membrane filters having a nominal pore size not greater than 0.45 µm whose effectiveness to retain microorganisms has been established. Cellulose nitrate filters, for example, are used for aqueous, oily, and weakly alcoholic solutions; and cellulose acetate filters, for example, are used for strongly alcoholic solutions. Specially adapted filters may be needed for certain products (e.g., for antibiotics).
The technique described below assumes that membranes about 50 mm in diameter will be used. If filters of a different diameter are used, the volumes of the dilutions and the washings should be adjusted accordingly. The filtration apparatus and membrane are sterilized by appropriate means. The apparatus is designed so that the solution to be examined can be introduced and filtered under aseptic conditions: it permits the aseptic removal of the membrane for transfer to the medium, or it is suitable for carrying out the incubation after adding the medium to the apparatus itself.
aqueous solutions
If appropriate, transfer a small quantity of a suitable, sterile diluent such as  Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration)
Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration) onto the membrane in the apparatus and filter. The diluent may contain suitable neutralizing substances and/or appropriate inactivating substances, for example, in the case of antibiotics.
onto the membrane in the apparatus and filter. The diluent may contain suitable neutralizing substances and/or appropriate inactivating substances, for example, in the case of antibiotics.
Transfer the contents of the container or containers to be tested to the membrane or membranes, if necessary, after diluting to the volume used in the Validation Test with the chosen sterile diluent, but using not less than the quantities of the product to be examined prescribed in Tables 2 and 3. Filter immediately. If the product has antimicrobial properties, wash the membrane not less than three times by filtering through it each time the volume of the chosen sterile diluent used in the Validation Test. Do not exceed a washing cycle of 5 times 200 mL, even if during validation it has been demonstrated that such a cycle does not fully eliminate the antimicrobial activity. Transfer the whole membrane to the culture medium or cut it aseptically into two equal parts, and transfer one half to each of two suitable media. Use the same volume of each medium as in the Validation Test. Alternatively, transfer the medium onto the membrane in the apparatus. Incubate the media for not less than 14 days.
soluble solids (other than antibiotics)
Use for each medium not less than the quantity prescribed in Tables 2 and 3 of the product dissolved in a suitable solvent, such as  Fluid A (Diluting and Rinsing Fluids for Membrane Filtration),
Fluid A (Diluting and Rinsing Fluids for Membrane Filtration), and proceed with the test as described above for Aqueous Solutions using a membrane appropriate to the chosen solvent.
and proceed with the test as described above for Aqueous Solutions using a membrane appropriate to the chosen solvent.
oils and oily solutions
Use for each medium not less than the quantity of the product prescribed in Tables 2 and 3. Oils and oily solutions of sufficiently low viscosity may be filtered without dilution through a dry membrane. Viscous oils may be diluted as necessary with a suitable sterile diluent such as isopropyl myristate shown not to have antimicrobial activity in the conditions of the test. Allow the oil to penetrate the membrane by its own weight, and then filter, applying the pressure or suction gradually. Wash the membrane at least three times by filtering through it each time about 100 mL of a suitable sterile solution such as  Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration)
Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration) containing a suitable emulsifying agent at a concentration shown to be appropriate in the validation of the test, for example polysorbate 80 at a concentration of 10 g per L
containing a suitable emulsifying agent at a concentration shown to be appropriate in the validation of the test, for example polysorbate 80 at a concentration of 10 g per L  (Fluid K)
(Fluid K) . Transfer the membrane or membranes to the culture medium or media, or vice versa, as described above for Aqueous Solutions, and incubate at the same temperatures and for the same times.
. Transfer the membrane or membranes to the culture medium or media, or vice versa, as described above for Aqueous Solutions, and incubate at the same temperatures and for the same times.
ointments and creams
Use for each medium not less than the quantities of the product prescribed in Tables 2 and 3. Ointments in a fatty base and emulsions of the water-in-oil type may be diluted to 1% in isopropyl myristate as described above, by heating, if necessary, to not more than 40 . In exceptional cases it may be necessary to heat to not more than 44
. In exceptional cases it may be necessary to heat to not more than 44 . Filter as rapidly as possible, and proceed as described above for Oils and Oily Solutions.
. Filter as rapidly as possible, and proceed as described above for Oils and Oily Solutions.
For prefilled syringes without attached sterile needles, expel the contents of each syringe into one or two separate membrane filter funnels or into separate pooling vessels prior to transfer. If a separate sterile needle is attached, directly expel the syringe contents as indicated above, and proceed as directed for Aqueous Solutions. Test the sterility of the needle, using Direct Inoculation under Validation Test.
solids for injection other than antibiotics
Constitute the test articles as directed on the label, and proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies. [note—If necessary, excess diluent can be added to aid in the constitution and filtration of the constituted test article.]
antibiotic solids for injection
Pharmacy Bulk Packages, < 5 g—
From each of 20 containers, aseptically transfer about 300 mg of solids, into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration), and mix; or constitute, as directed in the labeling, each of 20 containers and transfer a quantity of liquid or suspension, equivalent to about 300 mg of solids, into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A, and mix. Proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies.
Pharmacy Bulk Packages, 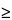 5 g—
From each of 6 containers, aseptically transfer about 1 g of solids into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A, and mix; or constitute, as directed in the labeling, each of 6 containers and transfer a quantity of liquid, equivalent to about 1 g of solids, into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A, and mix. Proceed as directed for Aqueous Solutions.
5 g—
From each of 6 containers, aseptically transfer about 1 g of solids into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A, and mix; or constitute, as directed in the labeling, each of 6 containers and transfer a quantity of liquid, equivalent to about 1 g of solids, into a sterile 500-mL conical flask, dissolve in about 200 mL of Fluid A, and mix. Proceed as directed for Aqueous Solutions.
antibiotic solids, bulks, and blends
Aseptically remove a sufficient quantity of solids from the appropriate amount of containers (see Table 2), mix to obtain a composite, equivalent to about 6 g of solids, and transfer to a sterile 500-mL conical flask. Dissolve in about 200 mL of Fluid A, and mix. Proceed as directed for Aqueous Solutions.
sterile aerosol products
For fluid products in pressurized aerosol form, freeze the containers in an alcohol-dry ice mixture at least at –20 for about 1 hour. If feasible, allow the propellant to escape before aseptically opening the container, and transfer the contents to a sterile pooling vessel. Add 100 mL of Fluid D to the pooling vessel, and mix gently. Proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies.
for about 1 hour. If feasible, allow the propellant to escape before aseptically opening the container, and transfer the contents to a sterile pooling vessel. Add 100 mL of Fluid D to the pooling vessel, and mix gently. Proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies.
devices with pathways labeled sterile
Aseptically pass not less than 10 pathway volumes of Fluid D through each device tested. Collect the fluids in an appropriate sterile vessel, and proceed as directed for Aqueous Solutions or Oils and Oily Solutions, whichever applies.
In the case of sterile, empty syringes, draw sterile diluent into the barrel through the sterile needle, if attached, or through a sterile needle attached for the purpose of the test, and express the contents into a sterile pooling vessel. Proceed as directed above.
Direct Inoculation of the Culture Medium
If the product to be examined has antimicrobial activity, carry out the test after neutralizing this with a suitable neutralizing substance or by dilution in a sufficient quantity of culture medium. When it is necessary to use a large volume of the product, it may be preferable to use a concentrated culture medium prepared in such a way that it takes into account the subsequent dilution. Where appropriate, the concentrated medium may be added directly to the product in its container.
oily liquids
Use media to which have been added a suitable emulsifying agent at a concentration shown to be appropriate in the validation of the test, for example polysorbate 80 at a concentration of 10 g per L.
ointments and creams
Prepare by diluting to about 1 in 10 by emulsifying with the chosen emulsifying agent in a suitable sterile diluent such as  Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration).
Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration). Transfer the diluted product to a medium not containing an emulsifying agent.
Transfer the diluted product to a medium not containing an emulsifying agent.
Incubate the inoculated media for not less than 14 days. Observe the cultures several times during the incubation period. Shake cultures containing oily products gently each day. However, when thioglycollate medium or other similar medium is used for the detection of anaerobic microorganisms, keep shaking or mixing to a minimum in order to maintain anaerobic conditions.
catgut and other surgical sutures for veterinarian use
Use for each medium not less than the quantities of the product prescribed in Tables 2 and 3. Open the sealed package using aseptic precautions, and remove three sections of the strand for each culture medium. Carry out the test on three sections, each 30-cm long, which have been cut off from the beginning, the center, and the end of the strand. Use whole strands from freshly opened cassette packs. Transfer each section of the strand to the selected medium. Use sufficient medium to cover adequately the material to be tested (20 mL to 150 mL).
Transfer a quantity of the product in the form of a dry solid (or prepare a suspension of the product by adding sterile diluent to the immediate container), corresponding to not less than the quantity indicated in Tables 2 and 3. Transfer the material so obtained to 200 mL of Fluid Thioglycollate Medium, and mix. Similarly, transfer the same quantity to 200 mL of Soybean–Casein Digest Medium, and mix. Proceed as directed above.
purified cotton, gauze, surgical dressings, and related articles
From each package of cotton, rolled gauze bandage, or large surgical dressings being tested, aseptically remove two or more portions of 100- to 500-mg each from the innermost part of the sample. From individually packaged, single-use materials, aseptically remove the entire article. Immerse the portions or article in each medium, and proceed as directed above.
sterile devices
Articles can be immersed intact or disassembled. To ensure that device pathways are also in contact with the media, immerse the appropriate number of units per medium in a volume of medium sufficient to immerse the device completely, and proceed as directed above. For extremely large devices, immerse those portions of the device that are to come into contact with the patient in a volume of medium sufficient to achieve complete immersion of those portions.
For catheters where the inside lumen and outside are required to be sterile, either cut them into pieces such that the medium is in contact with the entire lumen or fill the lumen with medium, and then immerse the intact unit.
OBSERVATION AND INTERPRETATION OF RESULTS
At intervals during the incubation period and at its conclusion, examine the media for macroscopic evidence of microbial growth. If the material being tested renders the medium turbid so that the presence or absence of microbial growth cannot be readily determined by visual examination, 14 days after the beginning of incubation transfer portions (each not less than 1 mL) of the medium to fresh vessels of the same medium, and then incubate the original and transfer vessels for not less than 4 days.
If no evidence of microbial growth is found, the product to be examined complies with the test for sterility. If evidence of microbial growth is found, the product to be examined does not comply with the test for sterility, unless it can be clearly demonstrated that the test was invalid for causes unrelated to the product to be examined. The test may be considered invalid only if one or more of the following conditions are fulfilled:
- The data of the microbiological monitoring of the sterility testing facility show a fault.
- A review of the testing procedure used during the test in question reveals a fault.
- Microbial growth is found in the negative controls.
- After determination of the identity of the microorganisms isolated from the test, the growth of this species (or these species) may be ascribed unequivocally to faults with respect to the material and or the technique used in conducting the sterility test procedure.
If the test is declared to be invalid, it is repeated with the same number of units as in the original test. If no evidence of microbial growth is found in the repeat test, the product examined complies with the test for sterility. If microbial growth is found in the repeat test, the product examined does not comply with the test for sterility.
APPLICATION OF THE TEST TO PARENTERAL PREPARATIONS, OPHTHALMIC, AND OTHER NONINJECTABLE PREPARATIONS REQUIRED TO COMPLY WITH THE TEST FOR STERILITY
When using the technique of membrane filtration, use, whenever possible, the whole contents of the container, but not less than the quantities indicated in Tables 2 and 3, diluting where necessary to about 100 mL with a suitable sterile solution, such as  Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration).
Fluid A (see Diluting and Rinsing Fluids for Membrane Filtration).
When using the technique of direct inoculation of media, use the quantities shown in Tables 2 and 3, unless otherwise justified and authorized. The tests for bacterial and fungal sterility are carried out on the same sample of the product to be examined. When the volume or the quantity in a single container is insufficient to carry out the tests, the contents of two or more containers are used to inoculate the different media.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 80