 711
711 DISSOLUTION
DISSOLUTION
This test is provided to determine compliance with the dissolution requirements where stated in the individual monograph for a tablet or capsule dosage form. Of the types of apparatus described herein, use the one specified in the individual monograph. Where the label states that an article is enteric-coated, and a dissolution or disintegration test that does not specifically state that it is to be applied to enteric-coated articles is included in the individual monograph, the test for
Delayed-Release Articles under
Drug Release  724
724
is applied unless otherwise specified in the individual monograph. For hard or soft gelatin capsules and gelatin-coated tablets that do not conform to the
Dissolution specification, repeat the test as follows. Where water or a medium with a pH of less than 6.8 is specified as the
Medium in the individual monograph, the same
Medium specified may be used with the addition of purified pepsin that results in an activity of 750,000 Units or less per 1000 mL. For media with a pH of 6.8 or greater, pancreatin can be added to produce not more than 1750 USP Units of protease activity per 1000 mL.
Apparatus 1—
The assembly consists of the following: a covered vessel made of glass or other inert, transparent material
1 ; a motor; a metallic drive shaft; and a cylindrical basket. The vessel is partially immersed in a suitable water bath of any convenient size or placed in a heating jacket. The water bath or heating jacket permits holding the temperature inside the vessel at 37 ± 0.5

during the test and keeping the bath fluid in constant, smooth motion. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smoothly rotating stirring element. Apparatus that permits observation of the specimen and stirring element during the test is preferable. The vessel is cylindrical, with a hemispherical bottom and with one of the following dimensions and capacities: for a nominal capacity of 1 L, the height is 160 mm to 210 mm and its inside diameter is 98 mm to 106 mm; for a nominal capacity of 2 L, the height is 280 mm to 300 mm and its inside diameter is 98 mm to 106 mm; and for a nominal capacity of 4 L, the height is 280 mm to 300 mm and its inside diameter is 145 mm to 155 mm. Its sides are flanged at the top. A fitted cover may be used to retard evaporation
2. The shaft is positioned so that its axis is not more than 2 mm at any point from the vertical axis of the vessel and rotates smoothly and without significant wobble. A speed-regulating device is used that allows the shaft rotation speed to be selected and maintained at the rate specified in the individual monograph, within ±4%.
Shaft and basket components of the stirring element are fabricated of stainless steel, type 316 or equivalent, to the specifications shown in
Figure 1.
Fig. 1. Basket Stirring Element
Unless otherwise specified in the individual monograph, use 40-mesh cloth. A basket having a gold coating 0.0001 inch (2.5 µm) thick may be used. The dosage unit is placed in a dry basket at the beginning of each test. The distance between the inside bottom of the vessel and the basket is maintained at 25 ± 2 mm during the test.
Apparatus 2—
Use the assembly from
Apparatus 1, except that a paddle formed from a blade and a shaft is used as the stirring element. The shaft is positioned so that its axis is not more than 2 mm at any point from the vertical axis of the vessel and rotates smoothly without significant wobble. The vertical center line of the blade passes through the axis of the shaft so that the bottom of the blade is flush with the bottom of the shaft. The paddle conforms to the specifications shown in
Figure 2.
Fig. 2. Paddle Stirring Element
The distance of 25 ± 2 mm between the blade and the inside bottom of the vessel is maintained during the test. The metallic or suitably inert, rigid blade and shaft comprise a single entity. A suitable two-part detachable design may be used provided the assembly remains firmly engaged during the test. The paddle blade and shaft may be coated with a suitable inert coating. The dosage unit is allowed to sink to the bottom of the vessel before rotation of the blade is started. A small, loose piece of nonreactive material such as not more than a few turns of wire helix may be attached to dosage units that would otherwise float. Other validated sinker devices may be used.
Apparatus Suitability Test—
Individually test 1 tablet of the USP Dissolution Calibrator, Disintegrating Type and 1 tablet of USP Dissolution Calibrator, Nondisintegrating Type, according to the operating conditions specified. The apparatus is suitable if the results obtained are within the acceptable range stated in the certificate for that calibrator in the apparatus tested.
Dissolution Medium—
Use the solvent specified in the individual monograph. If the
Dissolution Medium is a buffered solution, adjust the solution so that its pH is within 0.05 unit of the pH specified in the individual monograph.
[NOTE—Dissolved gases can cause bubbles to form, which may change the results of the test. In such cases, dissolved gases should be removed prior to testing.
3 ]
Time—
Where a single time specification is given, the test may be concluded in a shorter period if the requirement for minimum amount dissolved is met. If two or more times are specified, specimens are to be withdrawn only at the stated times, within a tolerance of ±2%.
Procedure for Capsules, Uncoated Tablets, and Plain Coated Tablets—
Place the stated volume of the
Dissolution Medium (±1%) in the vessel of the apparatus specified in the individual monograph, assemble the apparatus, equilibrate the
Dissolution Medium to 37 ± 0.5

, and remove the thermometer. Place 1 tablet or 1 capsule in the apparatus, taking care to exclude air bubbles from the surface of the dosage-form unit, and immediately operate the apparatus at the rate specified in the individual monograph. Within the time interval specified, or at each of the times stated, withdraw a specimen from a zone midway between the surface of the
Dissolution Medium and the top of the rotating basket or blade, not less than 1 cm from the vessel wall.
[NOTE—Replace the aliquots withdrawn for analysis with equal volumes of fresh
Dissolution Medium at 37

or, where it can be shown that replacement of the medium is not necessary, correct for the volume change in the calculation. Keep the vessel covered for the duration of the test, and verify the temperature of the mixture under test at suitable times.
] Perform the analysis as directed in the individual monograph
4. Repeat the test with additional dosage form units.
If automated equipment is used for sampling and the apparatus is modified, validation of the modified apparatus is needed to show that there is no change in the agitation characteristics of the test.
Where capsule shells interfere with the analysis, remove the contents of not fewer than 6 capsules as completely as possible, and dissolve the empty capsule shells in the specified volume of Dissolution Medium. Perform the analysis as directed in the individual monograph. Make any necessary correction. Correction factors greater than 25% of the labeled content are unacceptable.
Procedure for a Pooled Sample for Capsules, Uncoated Tablets, and Plain Coated Tablets—
Use this procedure where Procedure for a Pooled Sample is specified in the individual monograph. Proceed as directed under Procedure for Capsules, Uncoated Tablets, and Plain Coated Tablets. Combine equal volumes of the filtered solutions of the six or twelve individual specimens withdrawn, and use the pooled sample as the test solution. Determine the average amount of the active ingredient dissolved in the pooled sample.
Interpretation—
Unit Sample—
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient dissolved from the units tested conform to the accompanying
Acceptance Table. Continue testing through the three stages unless the results conform at either S
1 or S
2. The quantity,
Q, is the amount of dissolved active ingredient specified in the individual monograph, expressed as a percentage of the labeled content; the 5%, 15%, and 25% values in the
Acceptance Table are percentages of the labeled content so that these values and
Q are in the same terms.
Acceptance Table
| Stage |
Number Tested |
Acceptance Criteria |
| S1 |
6 |
Each unit is not less than Q + 5%. |
| S2 |
6 |
Average of 12 units (S1 + S2) is equal to or greater than Q, and no unit is less than Q 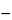 15%. 15%. |
| S3 |
12 |
Average of 24 units (S1 + S2 + S3) is equal to or greater than Q, not more than 2 units are less than Q 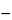 15%, and no unit is less than Q 15%, and no unit is less than Q 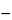 25%. 25%. |
Pooled Sample—
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient dissolved from the pooled sample conform to the accompanying
Acceptance Table for a Pooled Sample. Continue testing through the three stages unless the results conform at either S
1 or S
2. The quantity,
Q, is the amount of dissolved active ingredient specified in the individual monograph, expressed as a percentage of the labeled content.
Acceptance Table for a Pooled Sample
| Stage |
Number Tested |
Acceptance Criteria |
| S1 |
6 |
Average amount dissolved is not less than Q + 10%. |
| S2 |
6 |
Average amount dissolved (S1 + S2) is equal to or greater than Q + 5%. |
| S3 |
12 |
Average amount dissolved (S1 + S2 + S3) is equal to or greater than Q. |
1
The materials should not sorb, react, or interfere with the specimen being tested.
2
If a cover is used, it provides sufficient openings to allow ready insertion of the thermometer and withdrawal of specimens.
3
One method of deaeration is as follows: Heat the medium, while stirring gently, to about 41

, immediately filter under vacuum using a filter having a porosity of 0.45 µm or less, with vigorous stirring, and continue stirring under vacuum for about 5 minutes. Other validated deaeration techniques for removal of dissolved gases may be used.
4
If test specimens are filtered, use an inert filter that does not cause adsorption of the active ingredient or contain extractable substances that would interfere with the analysis.
Auxiliary Information—
Staff Liaison :
William E. Brown, Scientist
Expert Committee : (BPC05) Biopharmaceutics05
USP29–NF24 Page 2673
Pharmacopeial Forum : Volume No. 31(1) Page 198
Phone Number : 1-301-816-8380

